Abstract
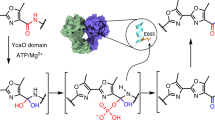
YcaO enzymes catalyze several post-translational modifications on peptide substrates, including thioamidation, which substitutes an amide oxygen with sulfur. Most predicted thioamide-forming YcaO enzymes are encoded adjacent to TfuA, which when present, is required for thioamidation. While activation of the peptide amide backbone is well established for YcaO enzymes, the function of TfuA has remained enigmatic. Here we characterize the TfuA protein involved in methyl-coenzyme M reductase thioamidation and demonstrate that TfuA catalyzes the hydrolysis of thiocarboxylated ThiS (ThiS-COSH), a proteinaceous sulfur donor, and enhances the affinity of YcaO toward the thioamidation substrate. We also report a crystal structure of a TfuA, which displays a new protein fold. Our structural and mutational analyses of TfuA have uncovered conserved binding interfaces with YcaO and ThiS in addition to revealing a hydrolase-like active site featuring a Ser–Lys catalytic pair.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
We declare that all the data supporting the findings of this study are available within the manuscript, Supplementary dataset or Supplementary Information. Plasmids are available upon request. X-ray crystallographic coordinates were deposited in the PDB under the code 6XP8. Source data are provided for Figs. 1–3 and Extended Data Figs. 6, 8 and 9. Public databases used in the study include Pfam (PF02624, PF07812, PF02597, PF00899, PF13407), UniProt, GenBank (AAB17515, AAB17513, WP_048176273, WP_048175617, WP_048175616, WP_048176081, WP_011023978) and the PDB (1RYJ, 2CU3, 6PEU, 4IRX, 3CNQ, 4PK9). The accession codes are also provided in the manuscript on the first occurrence.
References
El-Gebali, S. et al. The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427–D432 (2019).
UniProt Consortium, T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46, 2699 (2018).
Dunbar, K. L., Melby, J. O. & Mitchell, D. A. YcaO domains use ATP to activate amide backbones during peptide cyclodehydrations. Nat. Chem. Biol. 8, 569–575 (2012).
Burkhart, B. J., Schwalen, C. J., Mann, G., Naismith, J. H. & Mitchell, D. A. YcaO-dependent posttranslational amide activation: biosynthesis, structure, and function. Chem. Rev. 117, 5389–5456 (2017).
Dunbar, K. L. et al. Discovery of a new ATP-binding motif involved in peptidic azoline biosynthesis. Nat. Chem. Biol. 10, 823–829 (2014).
Dunbar, K. L., Tietz, J. I., Cox, C. L., Burkhart, B. J. & Mitchell, D. A. Identification of an auxiliary leader peptide-binding protein required for azoline formation in ribosomal natural products. J. Am. Chem. Soc. 137, 7672–7677 (2015).
Burkhart, B. J., Hudson, G. A., Dunbar, K. L. & Mitchell, D. A. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat. Chem. Biol. 11, 564–570 (2015).
Koehnke, J. et al. Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nat. Chem. Biol. 11, 558–563 (2015).
Montalbán-López, M. et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 38, 130–239 (2020).
Schwalen, C. J., Hudson, G. A., Kille, B. & Mitchell, D. A. Bioinformatic expansion and discovery of thiopeptide antibiotics. J. Am. Chem. Soc. 140, 9494–9501 (2018).
Nayak, D. D., Mahanta, N., Mitchell, D. A. & Metcalf, W. W. Post-translational thioamidation of methyl-coenzyme M reductase, a key enzyme in methanogenic and methanotrophic Archaea. eLife 6, e29218 (2017).
Mahanta, N., Liu, A., Dong, S., Nair, S. K. & Mitchell, D. A. Enzymatic reconstitution of ribosomal peptide backbone thioamidation. Proc. Natl Acad. Sci. USA 115, 3030–3035 (2018).
Watson, Z. L. et al. Structure of the bacterial ribosome at 2 Å resolution. eLife 9, e60482 (2020).
Mahanta, N., Szantai-Kis, D. M., Petersson, E. J. & Mitchell, D. A. Biosynthesis and chemical applications of thioamides. ACS Chem. Biol. 14, 142–163 (2019).
Izawa, M., Kawasaki, T. & Hayakawa, Y. Cloning and heterologous expression of the thioviridamide biosynthesis gene cluster from Streptomyces olivoviridis. Appl. Environ. Microbiol. 79, 7110–7113 (2013).
Frattaruolo, L., Lacret, R., Cappello, A. R. & Truman, A. W. A genomics-based approach identifies a thioviridamide-like compound with selective anticancer activity. ACS Chem. Biol. 12, 2815–2822 (2017).
Kjaerulff, L. et al. Thioholgamides: thioamide-containing cytotoxic RiPP natural products. ACS Chem. Biol. 12, 2837–2841 (2017).
Santos-Aberturas, J. et al. Uncovering the unexplored diversity of thioamidated ribosomal peptides in Actinobacteria using the RiPPER genome mining tool. Nucleic Acids Res. 47, 4624–4637 (2019).
Breil, B., Borneman, J. & Triplett, E. W. A newly discovered gene, tfuA, involved in the production of the ribosomally synthesized peptide antibiotic trifolitoxin. J. Bacteriol. 178, 4150–4156 (1996).
Ermler, U., Grabarse, W., Shima, S., Goubeaud, M. & Thauer, R. K. Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation. Science 278, 1457–1462 (1997).
Scheller, S., Goenrich, M., Boecher, R., Thauer, R. K. & Jaun, B. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465, 606–608 (2010).
Nayak, D. D. et al. Functional interactions between posttranslationally modified amino acids of methyl-coenzyme M reductase in Methanosarcina acetivorans. PLoS Biol. 18, e3000507 (2020).
Kahnt, J. et al. Post-translational modifications in the active site region of methyl-coenzyme M reductase from methanogenic and methanotrophic archaea. FEBS J. 274, 4913–4921 (2007).
Liu, Y., Sieprawska-Lupa, M., Whitman, W. B. & White, R. H. Cysteine is not the sulfur source for iron-sulfur cluster and methionine biosynthesis in the methanogenic archaeon Methanococcus maripaludis. J. Biol. Chem. 285, 31923–31929 (2010).
Lomans, B. P. et al. Isolation and characterization of Methanomethylovorans hollandica gen. nov., sp. nov., isolated from freshwater sediment, a methylotrophic methanogen able to grow on dimethyl sulfide and methanethiol. Appl. Environ. Microbiol. 65, 3641–3650 (1999).
Maupin-Furlow, J. A. Ubiquitin-like proteins and their roles in archaea. Trends Microbiol. 21, 31–38 (2013).
Lehmann, C., Begley, T. P. & Ealick, S. E. Structure of the Escherichia coli ThiS–ThiF complex, a key component of the sulfur transfer system in thiamin biosynthesis. Biochemistry 45, 11–19 (2006).
Dorrestein, P. C., Zhai, H., McLafferty, F. W. & Begley, T. P. The biosynthesis of the thiazole phosphate moiety of thiamin: the sulfur transfer mediated by the sulfur carrier protein this. Chem. Biol. 11, 1373–1381 (2004).
Rudolph, M. J., Wuebbens, M. M., Rajagopalan, K. V. & Schindelin, H. Crystal structure of molybdopterin synthase and its evolutionary relationship to ubiquitin activation. Nat. Struct. Biol. 8, 42–46 (2001).
Leidel, S. et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 458, 228–232 (2009).
Chen, M. et al. The [4Fe-4S] cluster of sulfurtransferase TtuA desulfurizes TtuB during tRNA modification in Thermus thermophilus. Commun. Biol. 3, https://doi.org/10.1038/s42003-020-0895-3 (2020).
Krishnamoorthy, K. & Begley, T. P. Protein thiocarboxylate-dependent methionine biosynthesis in Wolinella succinogenes. J. Am. Chem. Soc. 133, 379–386 (2011).
Dong, L.-B. et al. Biosynthesis of thiocarboxylic acid-containing natural products. Nat. Commun. 9, 2362 (2018).
Zallot, R., Oberg, N. & Gerlt, J. A. The EFI web resource for genomic enzymology tools: leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 58, 4169–4182 (2019).
Borrel, G. et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genomics 15, 679 (2014).
Dong, S.-H., Liu, A., Mahanta, N., Mitchell, D. A. & Nair, S. K. Mechanistic basis for ribosomal peptide backbone modifications. ACS Cent. Sci. 5, 842–851 (2019).
Krishnamoorthy, K. & Begley, T. P. Reagent for the detection of protein thiocarboxylates in the bacterial proteome: lissamine rhodamine B sulfonyl azide. J. Am. Chem. Soc. 132, 11608–11612 (2010).
Chen, B. et al. Fluorescent probe for highly selective and sensitive detection of hydrogen sulfide in living cells and cardiac tissues. Analyst 138, 946–951 (2013).
Yee, A. et al. An NMR approach to structural proteomics. Proc. Natl Acad. Sci. USA 99, 1825–1830 (2002).
Holm, L. DALI and the persistence of protein shape. Protein Sci. 29, 128–140 (2020).
Herrou, J. & Crosson, S. myo-inositol and d-ribose ligand discrimination in an ABC periplasmic binding protein. J. Bacteriol. 195, 2379–2388 (2013).
Ruan, B., London, V., Fisher, K. E., Gallagher, D. T. & Bryan, P. N. Engineering substrate preference in subtilisin: structural and kinetic analysis of a specificity mutant. Biochemistry 47, 6628–6636 (2008).
Ollis, D. L. et al. The α/β hydrolase fold. Protein Eng. Des. Sel. 5, 197–211 (1992).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Rydel, T. J. et al. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry 42, 6696–6708 (2003).
Mueller, E. G. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2, 185–194 (2006).
Ekici, Ö. D., Paetzel, M. & Dalbey, R. E. Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration. Protein Sci. 17, 2023–2037 (2008).
Miranda, H. V. et al. E1- and ubiquitin-like proteins provide a direct link between protein conjugation and sulfur transfer in archaea. Proc. Natl Acad. Sci. USA 108, 4417–4422 (2011).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D. Biol. Crystallogr. 67, 293–302 (2011).
Usón, I. & Sheldrick, G. M. An introduction to experimental phasing of macromolecules illustrated by SHELX; new autotracing features. Acta Crystallogr. Sect. Struct. Biol. 74, 106–116 (2018).
Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. Automated structure solution with autoSHARP. Methods Mol. Biol. Clifton NJ 364, 215–230 (2007).
Alharbi, E., Bond, P. S., Calinescu, R. & Cowtan, K. Comparison of automated crystallographic model-building pipelines. Acta Crystallogr. Sect. Struct. Biol. 75, 1119–1128 (2019).
Oeffner, R. D. et al. On the application of the expected log-likelihood gain to decision making in molecular replacement. Acta Crystallogr. Sect. Struct. Biol. 74, 245–255 (2018).
Casañal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. Publ. Protein Soc. 29, 1069–1078 (2020).
Kovalevskiy, O., Nicholls, R. A., Long, F., Carlon, A. & Murshudov, G. N. Overview of refinement procedures within REFMAC5: utilizing data from different sources. Acta Crystallogr. Sect. Struct. Biol. 74, 215–227 (2018).
Acknowledgements
We thank L. Zhu and J. Arrington for assistance with the NMR and nMS experiments, respectively. We thank X. Rui Guo from the Mitchell group for acquiring the high-resolution mass spectral data. This work was supported in part by National Institutes of Health grant nos. GM097142 (to D.A.M.), GM131347 (to S.K.N.) and the Alice Helm Graduate Research Excellence Fellowship in Microbiology (to A.L.). The Bruker UltrafleXtreme MALDI–TOF/TOF mass spectrometer was purchased in part with a grant from the National Institutes of Health (grant no. S10 RR027109 A).
Author information
Authors and Affiliations
Contributions
A.L. conducted the bioinformatics, kinetics and binding analyses. Y.S. and A.L. performed mutational and biochemical analyses of TfuA. The crystallographic work was performed by S-.H.D. under supervision by S.K.N. H.N.P. optimized the thiocarboxylate detection assay and assisted in variant generation. N.M. contributed to target selection, cloning, and protein purification. D.A.M. conceived of and supervised the overall project. A.L. wrote the first draft of the manuscript with input from D.A.M. and S.K.N., while all authors reviewed, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Wen Liu, Eugene Mueller and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 TfuA-associated RiPP structures and TfuA sequence analysis.
a, Representative thioamitide structures with thioamide moiety shown in red. b, A sequence similarity network (SSN) of the TfuA protein family PF07812 (n = 2,042 sequences) was generated with protein sequences 100% identical are conflated to a single node, and an alignment score of 60 (BLAST-P expectation value of 10-60) was used as the edge cut-off. Background is shaded based on taxonomy: archaea (orange), Proteobacteria (blue), Actinobacteria (pink), Cyanobacteria (purple), Firmicutes (green), and others (gray). Nodes representing TfuA proteins encoded within ten open-reading frames of a ThiS homolog are black. c, Gene neighborhood diagrams for selected methanogens encoding TfuA near ThiS. NCBI accession identifiers are given for the TfuA proteins.
Extended Data Fig. 2 Correlation analysis of TfuA and ThiS from methanogens with complete genomes.
a, Maximum-likelihood tree of YcaO proteins from methanogens (n = 111 sequences) with clades colored based on taxonomic group: Methanobacteria (purple), Methanococci (orange), Methanopyri (teal), Methanomicrobia (green), and Methanomassiliicoccales (magenta). The absence (open square) or presence (filled square) of tfuA and thiS within each genome is denoted. b, The cooccurrence table calculated for the correlation analysis between TfuA and ThiS. The P-value calculated using Fisher’s exact test is 1.6 × 10-12.
Extended Data Fig. 3 MALDI-TOF-MS analysis of MtThiS-COSH.
a, Top, MS spectrum of MtThiS (m/z 8,001 Da). Bottom, MS spectrum of MtThiS-COSH (thiocarboxylated C-terminus, m/z 8,017 Da). b, The same sample as above was digested with endoproteinase GluC. Shown is the spectral window surrounding the C-terminal peptide: VIRVIYGG.
Extended Data Fig. 4 High-resolution and tandem MS of the MtThiS-COSH C-terminal fragment.
a, High-resolution broadband spectrum of the C-terminal GluC peptide fragment of MtThiS-COSH. b, m/z 892.51 was subjected to CID (collision-induced dissociation) with assigned ions indicated in tabular form. c, Tandem mass spectrum (MS/MS) confirming the location of the +16 Da mass change to the C-terminus. CID also promotes the formal loss of H2S (obsv. Δm/z 33.9884 Da; calc. Δm/z 33.9877 Da) from the parent ion.
Extended Data Fig. 5 High-resolution and tandem MS of the thioamidated McrA peptide.
a, The thioamidated McrA peptide (GG-RLGFYGYDLQD) was characterized by HRMS. b, m/z 1,476.66 was subjected to CID with assigned ions indicated in tabular form. c, MS/MS spectrum confirming the location of the +16 Da mass change to the central Gly residue. Thioamide bond cleavage was not observed under the applied CID conditions.
Extended Data Fig. 6 TfuA catalyzes ThiS-COSH hydrolysis to generate sulfide.
Quantification of sulfide (a) and MtThiS-COSH (b) via fluorescence detection at different time points of the reaction between MtThiS-COSH (150 μM) and MtTfuA of various concentrations. Sulfide concentrations were measured via reaction with 7-amino-4-methylcoumarin followed by fluorescence quantification. ThiS-COSH concentrations were determined using the LRSA-based assay. Data are presented as mean values ± s.d. (n = 3 independent experiments). c, Fluorescence quantification of MtThiS-COSH at different time points after reaction with 50 μM MtYcaO, 5 mM ATP, and McrA peptide of various concentrations. Data are presented as mean values ± s.d. (n = 3 independent experiments).
Extended Data Fig. 7 McThiS 1H,15N-HMQC spectra upon MtTfuA titration.
a, An overlay of McThiS 1H,15N-HMQC spectra in the presence (red) and absence (black) of MtTfuA (2 equiv.). Residues with substantial chemical shift perturbations are labeled. b, Zoomed regions of the spectrum showing McThiS residues with the largest chemical shift changes. The MtTfuA: McThiS ratio varies from 0:1 to 6:1 equiv. (MtTfuA concentrations vary from 110 μM to 1.2 mM).
Extended Data Fig. 8 SDS-PAGE analysis of AzoYcaO co-purifying with His-AzoTfuA.
(a) A Coomassie-stained SDS-PAGE gel showing untagged AzoYcaO (BAI72909.1) and His6-AzoTfuA (BAI72908.1) from Azospirillum sp. B510 co-expressed heterologously and co-purified using Ni-NTA affinity chromatography. In-gel trypsin digestion and subsequent MS analysis was performed to confirm the identity of the suspected His6-AzoTfuA (b) and AzoYcaO (c) bands. MALDI-TOF mass spectra of the tryptic peptides are labeled with subscripts corresponding native amino acid sequence covered. The identified tryptic fragments cover ~40% of the total protein sequence for both proteins.
Extended Data Fig. 9 The presence of ATP alters the outcome of ThiS-COSH hydrolysis.
a, Fluorescence quantification of MtThiS-COSH (initially 200 μM) upon reaction with MtTfuA (3 μM), MtYcaO (3 μM), and McrA peptide (100 μM), in the presence (red circles) or absence (black squares) of ATP (5 mM). Data are presented as mean values ± s.d. (n = 3 independent experiments). An exponential decay model was used to fit the data. b, Fluorescence quantification of sulfide production for MtThiS-COSH control and reactions in panel a at 140 min. Individual data points (n = 3 independent experiments) and mean values (lines) are presented. c, MALDI-TOF mass spectra of the McrA peptide from reactions in panel a at 140 min.
Extended Data Fig. 10 Structural comparison of MaTfuA and proteins with partial structural similarity.
a, Overall structure of MaTfuA. b, Structure of myo-inositol-binding protein (PDB code: 4IRX) bound to its substrate (yellow stick) shows similarities with the α/β fold of MaTfuA (c) Structure of subtilisin BPN’ in complex with its prodomain (PDB code: 3CNQ) shows similarities with the α/β fold of MaTfuA. d, Close-up view of the superimposition between MaTfuA and 3CNQ shows the putative binding pocket for a peptide substrate. Residues that form the presumptive binding pocket are shown as tan (stick); the prodomain bound by subtilisin BPN’ is shown in blue (stick).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Figs. 1–23 and References.
Source data
Source Data Fig. 1
Raw data and model fitting statistics for Fig. 1b–e.
Source Data Fig. 2
Chemical shift data for Fig. 2b.
Source Data Fig. 3
Raw data and model fitting statistics for Fig. 3a,b; raw data and representative gel images for Fig. 3c.
Source Data Extended Data Fig. 6
Raw data and representative gel images for Extended Data Fig. 6a–c.
Source Data Extended Data Fig. 8
Uncropped gel image for Extended Data Fig. 8a.
Source Data Extended Data Fig. 9
Raw data and representative gel images for Extended Data Fig. 9a,b.
Rights and permissions
About this article
Cite this article
Liu, A., Si, Y., Dong, SH. et al. Functional elucidation of TfuA in peptide backbone thioamidation. Nat Chem Biol 17, 585–592 (2021). https://doi.org/10.1038/s41589-021-00771-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00771-0
This article is cited by
-
Core-dependent post-translational modifications guide the biosynthesis of a new class of hypermodified peptides
Nature Communications (2023)
-
Amino acid (acyl carrier protein) ligase-associated biosynthetic gene clusters reveal unexplored biosynthetic potential
Molecular Genetics and Genomics (2023)
-
Phylogenomic analysis of the diversity of graspetides and proteins involved in their biosynthesis
Biology Direct (2022)
-
A scalable platform to discover antimicrobials of ribosomal origin
Nature Communications (2022)