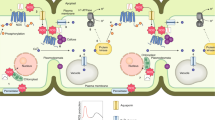
Abstract
How aerobic organisms exploit inevitably generated but potentially dangerous reactive oxygen species (ROS) to benefit normal life is a fundamental biological question. Locally accumulated ROS have been reported to prime stem cell differentiation. However, the underlying molecular mechanism is unclear. Here, we reveal that developmentally produced H2O2 in plant shoot apical meristem (SAM) triggers reversible protein phase separation of TERMINATING FLOWER (TMF), a transcription factor that times flowering transition in the tomato by repressing pre-maturation of SAM. Cysteine residues within TMF sense cellular redox to form disulfide bonds that concatenate multiple TMF molecules and elevate the amount of intrinsically disordered regions to drive phase separation. Oxidation triggered phase separation enables TMF to bind and sequester the promoter of a floral identity gene ANANTHA to repress its expression. The reversible transcriptional condensation via redox-regulated phase separation endows aerobic organisms with the flexibility of gene control in dealing with developmental cues.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data supporting the findings of this study are available within the paper and its Supplementary information files, or are available from the corresponding author upon reasonable request.
References
Irish, V. F. & Sussex, I. M. A fate map of the Arabidopsis embryonic shoot apical meristem. Development 115, 745–753 (1992).
Park, S. J., Eshed, Y. & Lippman, Z. B. Meristem maturation and inflorescence architecture-lessons from the Solanaceae. Curr. Opin. Plant Biol. 17, 70–71 (2014).
MacAlister, C. A. et al. Synchronization of the flowering transition by the tomato TERMINATING FLOWER gene. Nat. Genet. 44, 1393–1398 (2012).
Xu, C., Park, S. J., Van Eck, J. & Lippman, Z. B. Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev. 30, 2048–2061 (2016).
Iyer, L. M. & Aravind, L. ALOG domains: provenance of plant homeotic and developmental regulators from the DNA-binding domain of a novel class of DIRS1-type retroposons. Biol. Direct 7, 39 (2012).
Lippman, Z. B. et al. The making of a compound inflorescence in tomato and related nightshades. PLoS Biol. 6, 2424–2435 (2008).
Lei, Y., Su, S., He, L., Hu, X. & Luo, D. A member of the ALOG gene family has a novel role in regulating nodulation in Lotus japonicus. J. Integr. Plant Biol. 61, 463–477 (2019).
Takeda, S. et al. CUP-SHAPED COTYLEDON1 transcription factor activates the expression of LSH4 and LSH3, two members of the ALOG gene family, in shoot organ boundary cells. Plant J. 66, 1066–1077 (2011).
Zhao, L. et al. Overexpression of LSH1, a member of an uncharacterised gene family, causes enhanced light regulation of seedling development. Plant J. 37, 694–706 (2004).
Yoshida, A., Suzaki, T., Tanaka, W. & Hirano, H.-Y. The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc. Natl Acad. Sci. USA 106, 20103–20108 (2009).
Cho, E. & Zambryski, P. C. ORGAN BOUNDARY1 defines a gene expressed at the junction between the shoot apical meristem and lateral organs. Proc. Natl Acad. Sci. USA 108, 2154–2159 (2011).
Sato, D.-S., Ohmori, Y., Nagashima, H., Toriba, T. & Hirano, H.-Y. A role for TRIANGULAR HULL1 in fine-tuning spikelet morphogenesis in rice. Genes Genet. Syst. 89, 61–69 (2014).
Naramoto, S. et al. A conserved regulatory mechanism mediates the convergent evolution of plant shoot lateral organs. PLoS Biol. 17, e3000560 (2019).
Yoshida, A. et al. TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. Proc. Natl Acad Sci. USA 110, 767–772 (2013).
Chen, F. et al. Genome-wide identification and characterization of the ALOG gene family in Petunia. BMC Plant Biol. 19, 600 (2019).
Owusu-Ansah, E. & Banerjee, U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537–541 (2009).
Morimoto, H. et al. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 12, 774–786 (2013).
Zeng, J., Dong, Z., Wu, H., Tian, Z. & Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 36, 2844–2855 (2017).
Tsukagoshi, H., Busch, W. & Benfey, P. N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143, 606–616 (2010).
Yang, S. et al. ROS: the fine-tuner of plant stem cell fate. Trends Plant Sci. 23, 850–853 (2018).
Rampon, C., Volovitch, M., Joliot, A. & Vriz, S. Hydrogen peroxide and redox regulation of developments. Antioxidants 7, 159 (2018).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Foyer, C. H. & Noctor, G. Redox homeostasis and signaling in a higher-CO2 world. Annu. Rev. Plant Biol. 71, 157–182 (2020).
Paulsen, C. E. & Carroll, K. S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem. Biol. 5, 47–62 (2010).
Poole, L. B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 80, 148–157 (2015).
Zeida, A. et al. Catalysis of peroxide reduction by fast reacting protein thiols. Chem. Rev. 119, 10829–10855 (2019).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Kroschwald, S. et al. Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep. 23, 3327–3339 (2018).
Franzmann, T. M. et al. Phase separation of a yeast prion protein promotes cellular fitness. Science 359, eaao5654 (2018).
Riback, J. A. et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040.e19 (2017).
Kato, M. et al. Redox state controls phase separation of the yeast Ataxin-2 protein via reversible oxidation of its methionine-rich low-complexity domain. Cell 177, 711–721.e8 (2019).
Yang, Y. S. et al. Yeast Ataxin-2 forms an intracellular condensate required for the inhibition of TORC1 signaling during respiratory growth. Cell 177, 697–710.e17 (2019).
Zhang, G., Wang, Z., Du, Z. & Zhang, H. mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. Cell 174, 1492–1506.e22 (2018).
Wu, X., Cai, Q., Feng, Z. & Zhang, M. Liquid–liquid phase separation in neuronal development and synaptic signaling. Dev. Cell 55, 18–29 (2020).
Dunand, C., Crèvecoeur, M. & Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. N. Phytol. 174, 332–341 (2007).
Park, S. J., Jiang, K., Schatz, M. C. & Lippman, Z. B. Rate of meristem maturation determines inflorescence architecture in tomato. Proc. Natl Acad. Sci. USA 109, 639–644 (2012).
Doussiere, J. & Vignais, P. V. Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils. Factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Eur. J. Biochem. 208, 61–71 (1992).
Chen, X. et al. Apoplastic H2O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. N. Phytol. 211, 1266–1278 (2016).
Mei, Y., Chen, H., Shen, W., Shen, W. & Huang, L. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 17, 162 (2017).
Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 (2012).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Lin, Y., Protter, D. S. W., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Jiao, C.-J. et al. β-ODAP accumulation could be related to low levels of superoxide anion and hydrogen peroxide in Lathyrus sativus L. Food Chem. Toxicol. 49, 556–562 (2011).
Wang, L., Wang, X. & Wang, C. Protein disulfide–isomerase, a folding catalyst and a redox-regulated chaperone. Free Radic. Biol. Med. 83, 305–313 (2015).
Lyles, M. M. & Gilbert, H. F. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: dependence of the rate on the composition of the redox buffer. Biochemistry 30, 613–619 (1991).
Levy, Y. Y. & Dean, C. The transition to flowering. Plant Cell 10, 1973–1989 (1998).
Jones, D. P. Radical-free biology of oxidative stress. Am. J. Physiol.-Cell Physiol. 295, C849–C868 (2008).
Eck, J. V., Kirk, D. D. & Walmsley, A. M. In Agrobacterium Protocols. Methods in Molecular Biology Vol. 343 (ed. Wang, K.) (Humana Press, 2006); https://doi.org/10.1385/1-59745-130-4:459
Jackson, D. Molecular Plant Pathology: A Practical Approach (Oxford Univ. Press, 1992).
Xu, C. et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 47, 784–792 (2015).
Lin, R. et al. Transposase-derived transcription factors regulate light signaling in arabidopsis. Science 318, 1302–1305 (2007).
Gendrel, A.-V., Lippman, Z., Martienssen, R. & Colot, V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2, 213–218 (2005).
Shen, G. et al. Warfarin traps human vitamin K epoxide reductase in an intermediate state during electron transfer. Nat. Struct. Mol. Biol. 24, 69–76 (2017).
Li, H. et al. Crystal and solution structures of human protein-disulfide isomerase-like protein of the testis (PDILT) provide insight into its chaperone activity. J. Biol. Chem. 293, 1192–1202 (2018).
Shi, Y. et al. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and Type-A ARR genes in Arabidopsis. Plant Cell 24, 2578–2595 (2012).
Acknowledgements
We thank C.-C. Wang and L. Wang from the Institute of Biophysics, CAS for sharing PDI enzyme and valuable suggestions for related experiments, Z. B. Lippman (Cold Spring Harbor Laboratory), S. J. Park (Wonkwang University) and J. V. Eck (Boyce Thompson Institute) for sharing seeds, in situ images and the tomato transformation protocol, respectively. We thank J. Y. Li, Z. B. Lippman, J. Yang, K. Jiang and Q. Y. Wu for valuable comments and discussions; S. H. Yang and J. G. Li for sharing transformation vectors; Y. B. Tian, L. Fu, Z. Lu and S. Q. Jia for technical support and Y. F. Chen, W. X. Dong, Q. L. Guo, Y. Yu, Y. Xie, T. H. Zhang and X. Tong in the Xu laboratory for their help in this study. We are grateful to the staff from facilities of State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, CAS; Institute of Botany, CAS and Tsinghua University for technical assistance. This study was financially supported by the Key Research Program of Frontier Sciences of the Chinese Academy of Science (grant no. ZDBS-LY-SM021) and the Strategic Priority Research Program of Chinese Academy of Sciences (grant no. XDA24030503) to C.X., a National Key R&D grant (no. 2019YFA0508403) and a NSFC grant (no. 31871443) to P.L. and start-up funding from State Key Laboratory of Plant Genomics and Institute of Genetics and Developmental Biology to C.X. and a NSFC grant (no. 31900174) to X.H.
Author information
Authors and Affiliations
Contributions
C.X. and P.L. designed the research. X.H. performed in vivo condensate assays, chemical treatments, gene expression, immunoblots, ChIP–qPCR and transcriptional activity assays with help from N.X., S.C. and W. Li performed in vitro phase separation assays. S.C. performed EMSA, protein sedimentation assays and PDI treatments. X.H. and N.X. produced CRISPR mutants and transgenic lines with the help from X.Z. L.T. performed the yeast one-hybrid assay. Y. Zhang and W. Liu performed in situ hybridization and H2O2 staining. N.Y. performed mass spectrometric analysis. Y. Zou performed bioinformatic analysis. C.X. and P.L. wrote the paper with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Images and quantification data for hydrogen peroxide staining and redox chemical treatments.
a, DAB staining and stereoscope imaging showing the accumulation of H2O2 in tomato young leaves treated with or without H2O2 (10 mM) for 36 h. b,c, HPF staining (b) and quantitative data (c) showing the accumulation of H2O2 in the meristem treated with or without H2O2 (10 mM) for 48 h. (n=3). d,e, Stereoscope images (d) and quantitative data (e) comparing flowering transition indicated by leaf production until floral meristem stage transition from mock and H2O2 (10 mM) treated WT (upper) and tmf-2 (bottom). Leaf production is indicated by leaf number. L, Leaf. Scale bar, 100 μm. Data are presented as means (± s.d.). Sample size used for statistics of mock and H2O2 treatment for WT and tmf-2 is 19, 16, 43, 44, respectively. f, Phylogeny tree showing RBOH gene family in tomato. g, Expression of SlRBOH genes during meristem maturation of tomato. h, CRISPR/Cas9 gRNAs for targeting SlRBOH genes. i, DAB staining showing decreased H2O2 level in CRISPR mutant of slrboh1 slrboh2. Scale bar, 0.9 cm. j, Stereoscope images comparing flowering transition indicated by leaf production until floral meristem stage transition from WT and slrboh1 slrboh2 mutant, L, leaf. Scale bar, 100 μm. Three independent assays with similar results were carried out. In c and e, data are presented as means (± s.d.)(two-tailed t-test).
Extended Data Fig. 2 Droplet property and FRAP analysis of TMF-GFP condensates.
a, Amino acid sequence of TMF indicating IDRs, putative DNA binding domain and cysteine residues. b, Aspect ratios (maximal diameter/ minimal diameter) of droplets formed GFP-TMF. Gray ellipses show a guide to the eye of different aspect ratios. Totally, 202 droplets were measured for aspect ratio calculation. c, Quantification of TMF-GFP transfected tomato protoplast cells with or without condensates in nuclei. Three independent experiments were performed for quantification. Data are presented as (± s.d.) (n = 48). d,e, Image (d) and quantitative data (e) showing the recovery of TMF-GFP condensates after photobleaching in tomato protoplasts. The bleached (green line) event occurs at time = 0 s. The unbleached (blue line) was used as control. Quantitative data are representative of three independent photobleaching events. Data are presented as (± s.d.) (n = 3).
Extended Data Fig. 3 Quantification data and representative images for protein behavior of TMF and its mutated variants after redox chemicals treatment.
a, Quantification of integrated fluorescence density of the liquid-like droplets formed by GFP-TMF protein under various concentration combinations of H2O2 and DTT with constant protein concentration (25 μM). b,c, Representative confocal images (b) and quantification data (c) showing effects of TCEP treatment on droplet formation of GFP-TMF proteins. Protein concentration, 20 μM; Salt concentration, 25 mM. Scale bar, 20 μm. d, Schematic sedimentation assay for redox regulated phase separation. e,f, Immunoblotting (e) and quantification data (f) showing the distribution of TMF proteins between aqueous-solution/supernatant (S) and condensed liquid phase/pellet (P) fractions after H2O2 or DTT treatments. g, Schematics showing TMF variants with IDR or cysteine mutations. h, Quantification of integrated fluorescence density of the droplets formed by TMF variants with IDR or cysteine mutations. i, Immunoblot analysis showing the expression for TMF and variants with IDR or cysteine mutations in tomato protoplast. Actin serves as a loading control. In a,c,f,h, three technical replicates data are presented as means (± s.d.) (n = 3, two-tailed t-test).
Extended Data Fig. 4 Inter- and intramolecular disulfide bonds identified by LC-MS/MS.
a, LC-MS/MS spectrum of intramolecular and intermolecular disulfide bonds from normal TMF. b, Schematic diagrams showing the working model of PDI in different redox status. c, LC-MS/MS spectrum of intramolecular and intermolecular disulfide bonds from PDI treated TMF protein.
Extended Data Fig. 5 Representative colonies and quantification data of yeast one-hybrid assay.
a, Promoter regions upstream of the AN gene selected for yeast one hybrid assays in (b-d). b-d, Colony growth assessment (b) and quantification of β-galactosidase activity (c,d) in yeast one-hybrid assay. e, Promoter regions upstream of the AN gene selected for evaluating the ChIP enrichments. In c and d, three biological replicates data are presented as means (± s.d.) (n = 3, two-tailed t-test).
Extended Data Fig. 6 Quantification data for droplets, phase sedimentation assay and leaf production for flower transition.
a, Phase diagram showing droplets formed by GFP-TMF protein and Cy-3 labeled DNA fragments under various concentration combinations of H2O2 and DTT with constant protein concentration. Scale bar, 5 μm. b,c, Immunoblotting (b) and quantification data (c) showing the distribution of TMF-DNA complex between aqueous-solution/supernatant (S) and condensed liquid phase/pellet (P) fractions after H2O2 or DTT treatments. Three technical replicates data are presented. d, Western blot analysis showing expression of proteins in transgenic plants. The Wild-type (WT) plant sample served as a negative control, actin served as a loading control. e, Quantification of leaf number to flower transition on primary shoots. In c and e, data are means(± s.d.) (n = 3 for c, n = 8 for e, two-tailed t-test).
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Supplementary Video 1
Fusion process of two separated GFP–TMF droplets for Fig. 2f.
Supplementary Video 2
FRAP analysis of recombinantly expressed GFP–TMF droplets for Fig. 2g.
Supplementary Video 3
FRAP analysis of TMF–GFP condensates in tomato protoplasts for Extended Data Fig. 2d.
Supplementary Video 4
FRAP analysis of TMF–GFP condensates in the young leaf of 35S:TMF–GFP transgenic tomato plants for Fig. 2j.
Supplementary Video 5
FRAP analysis of droplets formed by GFP–TMF–DNA complex for Fig. 4e.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Huang, X., Chen, S., Li, W. et al. ROS regulated reversible protein phase separation synchronizes plant flowering. Nat Chem Biol 17, 549–557 (2021). https://doi.org/10.1038/s41589-021-00739-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00739-0
This article is cited by
-
Mechanism of composite passivators to reduce cadmium absorption and accumulation in Chinese cabbage on cadmium-polluted soil
Chemical and Biological Technologies in Agriculture (2024)
-
MolPhase, an advanced prediction algorithm for protein phase separation
The EMBO Journal (2024)
-
Liquid-liquid phase separation as a major mechanism of plant abiotic stress sensing and responses
Stress Biology (2023)
-
Redox homeostasis at SAM: a new role of HINT protein
Planta (2023)
-
Molecular regulation of tomato male reproductive development
aBIOTECH (2023)