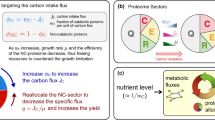
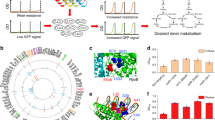
Abstract
Engineering resource allocation in biological systems is an ongoing challenge. Organisms allocate resources for ensuring survival, reducing the productivity of synthetic biology functions. Here we present a new approach for engineering the resource allocation of Escherichia coli by rationally modifying its transcriptional regulatory network. Our method (ReProMin) identifies the minimal set of genetic interventions that maximizes the savings in cell resources. To this end, we categorized transcription factors according to the essentiality of its targets and we used proteomic data to rank them. We designed the combinatorial removal of transcription factors that maximize the release of resources. Our resulting strain containing only three mutations, theoretically releasing 0.5% of its proteome, had higher proteome budget, increased production of an engineered metabolic pathway and showed that the regulatory interventions are highly specific. This approach shows that combining proteomic and regulatory data is an effective way of optimizing strains using conventional molecular methods.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA-seq data from this study have been deposited in NCBI’s Gene Expression Omnibus (GSE134335). Additional data is available from the corresponding author upon reasonable request.
Code availability
The code and data to run ReProMin can be found at: https://github.com/utrillalab/repromin
Change history
09 September 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Hutchison, C. A. et al. Design and synthesis of a minimal bacterial genome. Science 351, aad6253–aad6253 (2016).
Balikó, G. et al. in Synthetic Biology: Parts, Devices and Applications (eds Smolke, C., Lee, S. Y., Nielsen, J. & Stephanopoulos, G.) 49–80 (Wiley-VCH, 2018).
Ishihama, A. Prokaryotic genome regulation: multifactor promoters, multitarget regulators and hierarchic networks. FEMS Microbiol. Rev. 34, 628–645 (2010).
Ulrich, L. E. & Zhulin, I. B. The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 38, D401–D407 (2010).
Kitano, H. Biological robustness. Nat. Rev. Genet. 5, 826–837 (2004).
Isalan, M. et al. Evolvability and hierarchy in rewired bacterial gene networks. Nature 452, 840–845 (2008).
Mitchell, A. et al. Adaptive prediction of environmental changes by microorganisms. Nature 460, 220–224 (2009).
Tagkopoulos, I., Liu, Y.-C. & Tavazoie, S. Predictive behavior within microbial genetic networks. Science 320, 1313–1317 (2008).
Utrilla, J. et al. Global rebalancing of cellular resources by pleiotropic point mutations illustrates a multi-scale mechanism of adaptive evolution. Cell Syst. 2, 260–271 (2016).
O’Brien, E. J., Utrilla, J. & Palsson, B. O. Quantification and classification of E. coli proteome utilization and unused protein costs across environments. PLoS Comput. Biol. 12, e1004998 (2016).
Nikolados, E.-M., Weiße, A. Y., Ceroni, F. & Oyarzún, D. A. Growth defects and loss-of-function in synthetic gene circuits. ACS Synth. Biol. 8, 1231–1240 (2018).
Ceroni, F. et al. Burden-driven feedback control of gene expression. Nat. Methods 15, 387–393 (2018).
Darlington, A. P. S., Kim, J., Jiménez, J. I. & Bates, D. G. Dynamic allocation of orthogonal ribosomes facilitates uncoupling of co-expressed genes. Nat. Commun. 9, 695 (2018).
Lloyd, C. J. et al. COBRAme: a computational framework for genome-scale models of metabolism and gene expression. PLoS Comput. Biol. 14, e1006302 (2018).
Santos-Zavaleta, A. et al. RegulonDB v 10.5: tackling challenges to unify classic and high throughput knowledge of gene regulation in E. coli K-12. Nucleic Acids Res. 47, D212–D220 (2019).
Schmidt, A. et al. The quantitative and condition-dependent Escherichia coli proteome. Nat. Biotechnol. 34, 104–110 (2015).
Shen-Orr, S. S., Milo, R., Mangan, S. & Alon, U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 31, 64–68 (2002).
Kim, J., Darlington, A., Salvador, M., Utrilla, J. & Jiménez, J. I. Trade-offs between gene expression, growth and phenotypic diversity in microbial populations. Curr. Opin. Biotechnol. 62, 29–37 (2020).
Turner, P. C. et al. YqhC regulates transcription of the adjacent Escherichia coli genes yqhD and dkgA that are involved in furfural tolerance. J. Ind. Microbiol. Biotechnol. 38, 431–439 (2011).
Yakovleva, G. M., Kim, S. K. & Wanner, B. L. Phosphate-independent expression of the carbon-phosphorus lyase activity of Escherichia coli. Appl. Microbiol. Biotechnol. 49, 573–578 (1998).
Gyorgy, A. et al. Isocost lines describe the cellular economy of genetic circuits. Biophys. J. 109, 639–646 (2015).
Bienick, M. S., Young, K. W., Klesmith, J. R., Detwiler, E. E. & Tomek, K. J. The interrelationship between promoter strength, gene expression, and growth rate. PLoS ONE 9, 109105 (2014).
Durán, N. et al. Advances in Chromobacterium violaceum and properties of violacein-Its main secondary metabolite: a review. Biotechnol. Adv. 34, 1030–1045 (2016).
Long, C. P., Gonzalez, J. E., Feist, A. M., Palsson, B. O. & Antoniewicz, M. R. Fast growth phenotype of E. coli K-12 from adaptive laboratory evolution does not require intracellular flux rewiring. Metab. Eng. 44, 100–107 (2017).
Baumstark, R. et al. The propagation of perturbations in rewired bacterial gene networks. Nat. Commun. 6, 10105 (2015).
Posfai, G. et al. Emergent properties of reduced-genome Escherichia coli. Science 312, 1044–1046 (2006).
Hidalgo, D. & Utrilla, J. in Minimal Cells: Design, Construction, Biotechnological Applications (eds Lara, A. & Gosset, G.) 211–230 (Springer International Publishing, 2020); https://doi.org/10.1007/978-3-030-31897-0_8
Fang, X. et al. Global transcriptional regulatory network for Escherichia coli robustly connects gene expression to transcription factor activities. Proc. Natl Acad. Sci. USA 114, 10286–10291 (2017).
Ibarra-Arellano, M. A., Campos-González, A. I., Treviño-Quintanilla, L. G., Tauch, A. & Freyre-González, J. A. Abasy Atlas: a comprehensive inventory of systems, global network properties and systems-level elements across bacteria. Database 2016, baw089 (2016).
Sastry, A. V. et al. The Escherichia coli transcriptome mostly consists of independently regulated modules. Nat. Commun. 10, 1–14 (2019).
Couto, J. M., McGarrity, A., Russell, J. & Sloan, W. T. The effect of metabolic stress on genome stability of a synthetic biology chassis Escherichia coli K12 strain. Microb. Cell Fact. 17, 8 (2018).
Choe, D. et al. Adaptive laboratory evolution of a genome-reduced Escherichia coli. Nat. Commun. 10, 935 (2019).
Yang, L. et al. Principles of proteome allocation are revealed using proteomic data and genome-scale models. Sci. Rep. 6, 36734 (2016).
McCloskey, D. et al. Evolution of gene knockout strains of E. coli reveal regulatory architectures governed by metabolism. Nat. Commun. 9, 3796 (2018).
Nilsson, A., Nielsen, J. & Palsson, B. O. Metabolic models of protein allocation call for the kinetome. Cell Syst. 5, 538–541 (2017).
Klein-Marcuschamer, D., Santos, C. N. S., Yu, H. & Stephanopoulos, G. Mutagenesis of the bacterial RNA polymerase alpha subunit for improvement of complex phenotypes. Appl. Environ. Microbiol. 75, 2705–2711 (2009).
Yang, L. et al. Systems biology definition of the core proteome of metabolism and expression is consistent with high-throughput data. Proc. Natl Acad. Sci. USA 112, 10810–10815 (2015).
Kato, J.-I. & Hashimoto, M. Construction of consecutive deletions of the Escherichia coli chromosome. Mol. Syst. Biol. 3, 1–7 (2007).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Yamamoto, N. et al. Update on the Keio collection of Escherichia coli single-gene deletion mutants. Mol. Syst. Biol. 5, 335 (2009).
O’Brien, E. J., Lerman, J. A., Chang, R. L., Hyduke, D. R. & Palsson, B. Ø. Genome-scale models of metabolism and gene expression extend and refine growth phenotype prediction. Mol. Syst. Biol. 9, 693 (2013).
Orth, J. D. & Palsson, B. Gap-filling analysis of the iJO1366 Escherichia coli metabolic network reconstruction for discovery of metabolic functions. BMC Syst. Biol. 6, 30 (2012).
Monk, J. M. et al. iML1515, a knowledgebase that computes Escherichia coli traits. Nat. Biotechnol. 35, 904–908 (2017).
Ebrahim, A., Lerman, J. A., Palsson, B. O. & Hyduke, D. R. COBRApy: constraints-based reconstruction and analysis for python. BMC Syst. Biol. 7, 74 (2013).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Miller, J. H. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria (Cold Spring Harbor Laboratory Press, 1992).
St-Pierre, F. et al. One-step cloning and chromosomal integration of DNA. ACS Synth. Biol. 2, 537–541 (2013).
Swain, P. S. et al. Inferring time derivatives including cell growth rates using Gaussian processes. Nat. Commun. 7, 13766 (2016).
Acknowledgements
We thank E. Marquez-Zavala and C. Lloyd for metabolism and gene-expression model simulations support. Y. Castillo-Franco and C. F. Mendez-Cruz for computational support and G. Hernandez-Chavez, H. King, M. Hughes and A. Sicilia for technical support. We acknowledge the funding provided by UNAM–DGAPA-PAPIIT projects IA200716 and IA201518. Newton advanced Fellowship Project NA 160328. J.J. and J.K. acknowledge the support received from the Biology and Biotechnology Research Council (grant nos. BB/M009769/1 and BB/T011289/1) and European Union’s Horizon 2020 research and innovation program for the project P4SB (grant agreement no. 633962). G.L.P. acknowledges the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM), and the PhD scholarship 434655 from CONACyT.
Author information
Authors and Affiliations
Contributions
J.U. and G.L.P. designed ReProMin. G.L.P. developed computational methods and performed data analysis. G.L.P. and J.S.M.H. carried out experiments. G.L.P., J.K. and J.I.J. analyzed flow cytometry experiments, isocost lines and violacein production. J.U. supervised the study. J.U., G.L.P. and J.I.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.U. and G.L.P. are inventors in a MX patent application filled by UNAM.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ME-model simulations and proteome sector response to reducing the unmodeled protein fraction (UPF).
The ME-model iJL1678b-ME was used to simulate the effect of the reduction of the UPF and different expression levels of an unused recombinant model protein (GFP) (see methods). Similar to the maintenance energy coefficient, the hedging proteome and other non-growth related (thus not modeled) functions are accounted for in ME-models as a part of the UPF. Each panel shows a, growth rate and the corresponding fraction of each proteome sector b, core sector and the alternative element dependent sector: c, the carbon sector d, the nitrogen sector e, the phosphate sector f, the sulphur sector g, the non-ME sector h, the recombinant sector, comprised by the maximum attained GFP expression, i, the other sector (non-classified) and j, the UPF sector. The simulation shows an increased availability of cellular resources for recombinant protein production by reducing the UPF.
Extended Data Fig. 2 ReProMin proteome liberation landscapes corresponding to the UT case.
Potential proteome liberation landscape corresponding to a, Galactose, b, Acetate, c, Glycerol + casAA and d, Rich Medium (LB).
Extended Data Fig. 3 Regulatory subnetwork of ReProMin predicted gene targets.
Subnetwork corresponding to a, ST case PYC mutant and b, UT case PFC mutant; blue circles represent predicted silenced targets, yellow circles predicted induced targets and gray circles genes with no proteomic coverage; size of the circles is proportional to the PL of the target.
Extended Data Fig. 4 Transcriptomic analysis of the UT case designed strain.
a, Correlation plot for PFC and WT strains transcripts. Green squares represent the three deleted TFs. b, Volcano plot showing differential gene expression. In both cases, statistically significant genes are highlighted (blue – downregulated, yellow—upregulated) (log2 Fold Change ≥ 1 or ≤−1, P ≤ 0.01, n = 2). c, Integration of transcriptomics with computational tool predictions. The size of the circle corresponds to the fold change of each target (the largest circles represent fully silenced genes), in all cases blue circles represent targets releasing resources (down regulated), yellow circles represent targets generating burden (upregulated) and grey circles targets that were not found expressed. d, Accuracy of computational tool predictions based on RNAseq data. Yellow circles represent wrong predictions, blue circles represent accurate predictions and grey circles represent unmapped predictions (expression was not detected).
Extended Data Fig. 5 Phenotypic evaluation generated strains based on glucose ReProMin predictions (UT and ST cases) and control.
Growth on different carbon source supplemented M9 medium and rich medium (LB). a-d, shows max growth rate and f-i, shows max O.D. Points represent the Gaussian fitted value ± 2 s.d. for n = 9.
Extended Data Fig. 6 Metabolic burden evaluation of strains based on glucose ReProMin predictions (UT and ST cases) and control.
Metabolic burden while carrying empty, circuit plasmid and induced circuit plasmid, a, shows max growth rate and b, shows max O.D. Points represent the Gaussian fitted value ± 2 s.d. for n = 9.
Extended Data Fig. 7 Synthetic circuit characterization in glucose M9 medium.
Isocost lines showing mean fluorescence per cell measured by flow cytometry during balanced growth (~5 h). Points represent the Gaussian fitted fluorescence value ± 2 s.d. for n = 9 of red reporter (x axis) plotted against the green reporter (y axis) in an increasing inducer concentration (0, 2.5, 5, 20 nM AHL). A linear regression was used to fit the points to a line.
Extended Data Fig. 8 Isocost lines during balanced growth (~5 h).
Isocost lines of the generated mutant strains for two growth conditions: a, Glucose M9 medium and b, Rich medium. Left: absolute fluorescence, Right: normalized fluorescence. Points represent the Gaussian fitted fluorescence value ± 2 s.d. for n = 9 of red reporter (x axis) plotted against the green reporter (y axis) in an increasing inducer concentration (0, 2.5, 5, 20 nM AHL). A linear regression was used to fit the points to a line.
Extended Data Fig. 9 Isocost lines during stationary phase (~22 hrs).
Isocost lines of the generated mutant strains for two growth conditions: a, Glucose M9 medium and b, Rich medium. Left: absolute fluorescence, Right: normalized fluorescence Points represent the Gaussian fitted fluorescence value ± 2 s.d. for n = 9 of red reporter (x axis) plotted against the green reporter (y axis) in an increasing inducer concentration (0, 2.5, 5, 20 nM AHL). A linear regression was used to fit the points to a line.
Extended Data Fig. 10 Violacein production characterization.
a, Protein normalized violacein production using 2 g/L tryptophan in the presence of AHL (20 nM) (mean ± s.d., n = 9). b, Total violacein production without adding tryptophan after 24 h in the presence of increasing inducer (AHL) concentrations (mean ± s.d., n = 9). Asterisks *, ** and *** denote significant differences between WT and PFC using a two-tailed unpaired Student’s t-test. The following P values were obtained for normalized violacein production: 1 h, P = 0.0003; 2 h, P = 0.5647; 6 h, P < 0.0001. The following P values were obtained for violacein production with different AHL concentrations: No AHL, P = 0.0599; 1.25 nM, P = 0.0146; 2.5 nM, P = 0.0021; 5 nM, P < 0.0001; 10 nM, P = 0.0014; 20 nM, P = 0.0005.
Supplementary information
Supplementary Tables
Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Lastiri-Pancardo, G., Mercado-Hernández, J.S., Kim, J. et al. A quantitative method for proteome reallocation using minimal regulatory interventions. Nat Chem Biol 16, 1026–1033 (2020). https://doi.org/10.1038/s41589-020-0593-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0593-y
This article is cited by
-
Enhancing Escherichia coli abiotic stress resistance through ornithine lipid formation
Applied Microbiology and Biotechnology (2024)
-
Resource-aware construct design in mammalian cells
Nature Communications (2023)
-
Glucose consumption rate-dependent transcriptome profiling of Escherichia coli provides insight on performance as microbial factories
Microbial Cell Factories (2022)
-
Understanding and mathematical modelling of cellular resource allocation in microorganisms: a comparative synthesis
BMC Bioinformatics (2021)
-
Picking the right metaphors for addressing microbial systems: economic theory helps understanding biological complexity
International Microbiology (2021)