Abstract
The orphan nuclear receptor Nurr1 is critical for the development, maintenance and protection of midbrain dopaminergic (mDA) neurons. Here we show that prostaglandin E1 (PGE1) and its dehydrated metabolite, PGA1, directly interact with the ligand-binding domain (LBD) of Nurr1 and stimulate its transcriptional function. We also report the crystallographic structure of Nurr1-LBD bound to PGA1 at 2.05 Å resolution. PGA1 couples covalently to Nurr1-LBD by forming a Michael adduct with Cys566, and induces notable conformational changes, including a 21° shift of the activation function-2 helix (H12) away from the protein core. Furthermore, PGE1/PGA1 exhibit neuroprotective effects in a Nurr1-dependent manner, prominently enhance expression of Nurr1 target genes in mDA neurons and improve motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse models of Parkinson’s disease. Based on these results, we propose that PGE1/PGA1 represent native ligands of Nurr1 and can exert neuroprotective effects on mDA neurons, via activation of Nurr1’s transcriptional function.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Evans, R. M. & Mangelsdorf, D. J. Nuclear receptors, RXR, and the big bang. Cell 157, 255–266 (2014).
Kliewer, S. A., Lehmann, J. M. & Willson, T. M. Orphan nuclear receptors: shifting endocrinology into reverse. Science 284, 757–760 (1999).
Kurakula, K., Koenis, D. S., van Tiel, C. M. & de Vries, C. J. NR4A nuclear receptors are orphans but not lonesome. Biochim Biophys. Acta 1843, 2543–2555 (2014).
Pearen, M. A. & Muscat, G. E. Minireview: nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol. Endocrinol. 24, 1891–1903 (2010).
Zetterstrom, R. H. et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science 276, 248–250 (1997).
Kadkhodaei, B. et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J. Neurosci. 29, 15923–15932 (2009).
Saijo, K. et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 (2009).
Chu, Y. et al. Nurr1 in Parkinson’s disease and related disorders. J. Comp. Neurol. 494, 495–514 (2006).
Moran, L. B. et al. Analysis of alpha-synuclein, dopamine and parkin pathways in neuropathologically confirmed Parkinsonian nigra. Acta Neuropathol. 113, 253–263 (2007).
Decressac, M., Volakakis, N., Bjorklund, A. & Perlmann, T. NURR1 in Parkinson disease–from pathogenesis to therapeutic potential. Nat. Rev. Neurol. 9, 629–636 (2013).
Kim, C. H., Leblanc, P. & Kim, K. S. 4-amino-7-chloroquinoline derivatives for treating Parkinson’s disease: implications for drug discovery. Expert Opin. Drug Discov. 11, 337–341 (2016).
Kim, K. S. Toward neuroprotective treatments of Parkinson’s disease. Proc. Natl Acad. Sci. USA 114, 3795–3797 (2017).
Wang, Z. et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423, 555–560 (2003).
Kagaya, S. et al. Prostaglandin A2 acts as a transactivator for NOR1 (NR4A3) within the nuclear receptor superfamily. Biol. Pharm. Bull. 28, 1603–1607 (2005).
Lakshmi, S. P., Reddy, A. T., Banno, A. & Reddy, R. C. Molecular, chemical, and structural characterization of prostaglandin A2 as a novel agonist for Nur77. Biochem. J. 476, 2757–2767 (2019).
Vinayavekhin, N. & Saghatelian, A. Discovery of a protein-metabolite interaction between unsaturated fatty acids and the nuclear receptor Nur77 using a metabolomics approach. J. Am. Chem. Soc. 133, 17168–17171 (2011).
de Vera, I. M. et al. Identification of a binding site for unsaturated fatty acids in the orphan nuclear receptor Nurr1. ACS Chem. Biol. 11, 1795–1799 (2016).
de Vera, I. M. S. et al. Defining a canonical ligand-binding pocket in the orphan nuclear receptor Nurr1. Structure 27, 66–77 e65 (2019).
Chintharlapalli, S. et al. Activation of Nur77 by selected 1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem. 280, 24903–24914 (2005).
Zhan, Y. et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 4, 548–556 (2008).
Kim, C. H. et al. Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc. Natl Acad. Sci. USA 112, 8756–8761 (2015).
Andersen, N. H. Dehydration of prostaglandins: study by spectroscopic method. J. Lipid Res. 10, 320–325 (1969).
Itoh, T. et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct. Mol. Biol. 15, 924–931 (2008).
Codina, A. et al. Identification of a novel co-regulator interaction surface on the ligand binding domain of Nurr1 using NMR footprinting. J. Biol. Chem. 279, 53338–53345 (2004).
Aarnisalo, P., Kim, C. H., Lee, J. W. & Perlmann, T. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J. Biol. Chem. 277, 35118–35123 (2002).
Zhan, Y. Y. et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat. Chem. Biol. 8, 897–904 (2012).
Li, L. et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat. Chem. Biol. 11, 339–346 (2015).
Wang, W. J. et al. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat. Chem. Biol. 10, 133–140 (2014).
Wang, W. J. et al. Induction of autophagic death in cancer cells by agonizing TR3 and attenuating Akt2 activity. Chem. Biol. 22, 1040–1051 (2015).
Furuyashiki, T. & Narumiya, S. Stress responses: the contribution of prostaglandin E(2) and its receptors. Nat. Rev. Endocrinol. 7, 163–175 (2011).
Carrasco, E., Casper, D. & Werner, P. PGE(2) receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE(2) neurotoxicity. J. Neurosci. Res. 85, 3109–3117 (2007).
Parga, J. A. et al. Prostaglandin EP2 receptors mediate mesenchymal stromal cell-neuroprotective effects on dopaminergic neurons. Mol. Neurobiol. 55, 4763–4776 (2018).
Xu, H. et al. The MDM2-binding region in the transactivation domain of p53 also acts as a Bcl-X(L)-binding motif. Biochemistry 48, 12159–12168 (2009).
Becker, W., Bhattiprolu, K. C., Gubensak, N. & Zangger, K. Investigating protein-ligand interactions by solution nuclear magnetic resonance spectroscopy. Chem. Phys. Chem. 19, 895–906 (2018).
Choi, H. K., Won, L., Roback, J. D., Wainer, B. H. & Heller, A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc. Natl Acad. Sci. USA 89, 8943–8947 (1992).
Gao, L., Zhou, W., Symmes, B. & Freed, C. R. Re-cloning the N27 dopamine cell line to improve a cell culture model of Parkinson’s disease. PLoS ONE 11, e0160847 (2016).
af Forselles, K. J. et al. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. Br. J. Pharmacol. 164, 1847–1856 (2011).
Bergstroem, S. & Samuelsson, B. Prostaglandins. Annu. Rev. Biochem. 34, 101–108 (1965).
Higdon, A., Diers, A. R., Oh, J. Y., Landar, A. & Darley-Usmar, V. M. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem. J. 442, 453–464 (2012).
Qin, Z. H. et al. Prostaglandin A(1) protects striatal neurons against excitotoxic injury in rat striatum. J. Pharmacol. Exp. Ther. 297, 78–87 (2001).
Wang, X. et al. Prostaglandin A1 inhibits rotenone-induced apoptosis in SH-SY5Y cells. J. Neurochem. 83, 1094–1102 (2002).
Zhang, H. L. et al. Neuroprotective effects of prostaglandin A1 in animal models of focal ischemia. Brain Res. 1039, 203–206 (2005).
Bruning, J. M. et al. Covalent modification and regulation of the nuclear receptor nurr1 by a dopamine metabolite. Cell Chem. Biol. 26, 674–685 e676 (2019).
de Urquiza, A. M. et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290, 2140–2144 (2000).
Janowski, B. A., Willy, P. J., Devi, T. R., Falck, J. R. & Mangelsdorf, D. J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383, 728–731 (1996).
Powell, W. S. 15-Deoxy-delta12,14-PGJ2: endogenous PPARgamma ligand or minor eicosanoid degradation product? J. Clin. Invest. 112, 828–830 (2003).
Battye, T. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D. 67, 271–281 (2011).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D. 62, 72–82 (2006).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D. 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D. 67, 355–367 (2011).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 60, 2126–2132 (2004).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 66, 213–221 (2010).
Waudby, C. A., Ramos, A., Cabrita, L. D. & Christodoulou, J. Two-dimensional NMR lineshape analysis. Sci. Rep. 6, 24826 (2016).
Leblanc, P. et al. Production of Nurr-1 specific polyclonal antibodies free of cross-reactivity against its close homologs, Nor1 and Nur77. J. Vis. Exp. 102, e52963 (2015).
Kim, W. et al. miR-126 contributes to Parkinson’s disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiol. Aging 35, 1712–1721 (2014).
Chen, S. H., Oyarzabal, E. A. & Hong, J. S. Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods Mol. Biol. 1041, 231–240 (2013).
Acknowledgements
We thank various members of the molecular neurobiology laboratory past and present who participated in the project. In particular, we thank B.-S. Han, H.-Y. Jung, J. Lee and J. Sung Koh for technical assistance. We also acknowledge the contribution of the scientists and staff on the PXII and PXIII (Paul Scherrer Institute, Switzerland) beamlines for their expert assistance during crystal data collection. This work was supported by NIH grant nos. NS070577 and NS084869 (to K.-S.K.), NRF-2018M3A9B5023055 grant (to C.-H.K.), Ministry of Education Singapore AcRF Tier 2 Grant (no. ARC55/16) and Tang Tieng See Advancement Fund (to H.S.Y.), and National Medical Research Council, Singapore (grant no. TCR/013-NNI/2014; to K.L.L. and H.S.Y.).
Author information
Authors and Affiliations
Contributions
K.-S.K. and H.S.Y. initiated and supervised the project. S.R., Y.J., C.H.K. and W.K. were responsible for the overall design and performance of experiments. S.R., H.T.T., H.Y., C.K., A.S., J.L., J.Y.Y., S.B., H.Y., C.K., X.L., G.G. and K.L.L. performed and analyzed structural studies. Y.J., C.H.K., W.K, J.J., B.S., M.F., Y.K., D.H., H.M.P., S.F.O. and C.H.L. performed and analyzed functional and biological studies. K.-S.K., H.S.Y., S.R., Y.J., C.H.K., W.K. and G.A.P. analyzed the data and wrote the paper. All authors contributed to the discussion and final approval of the paper.
Corresponding authors
Ethics declarations
Competing interests
H.S.Y. is a nonexecutive director of Lifex Biolabs. The remaining authors have no competing interests to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
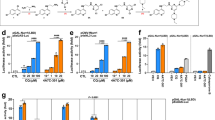
Extended Data Fig. 1 Identification of PGE1 from brain tissue extract.
(a) Isolation of endogenous ligand candidates using a combination of boiling, acetone precipitation, and ultrafiltration (3,000 molecular weight cut-off). Fractions were monitored for their Nurr1-enhancing activity using a cell-based luciferase assay system. Nurr1-enhancing activity was unaffected by boiling and acetone precipitation. n = 3 independent experiments, Data are presented as mean ± s.d. (b) Following ultrafiltration, filtrates were fractionated by HPLC column C-18 and each fraction was assayed for Nurr1-activating activities. Fraction 5 contained the most activity and thus was used for the mass spectrometry (MS) analysis. n = 3 independent experiments, Data are presented as mean ± s.d. (c) Candidate compounds that are tested after identification by an ultra-performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC–qTOF-MS/MS).
Extended Data Fig. 2 Direct binding of PGE1 and PGA1 to Nurr1-LBD.
(a–f) Molecular interaction of Nurr1-LBD with PGE1 (a and b) and PGA1 (c and d) studied using 2D HSQC NMR titration experiments with uniformly 15N-labeled Nurr1-LBD. (a) Close-up view of a section of the overlay of free Nurr1-LBD (red) and Nurr1-LBD with PGE1 (1:4, green; 1:10, blue), with residues showing chemical shift perturbations (with arrows) or intensity changes (boxed in red) labelled. (b) Selected residues were mapped on the crystal structure of Nurr1-LBD (PDB: 1OVL) in surface representation, with a close-up section (as inset) showing affected helices H4, H11 and H12, with amino acid residues indicated. (c) Close-up view of a section of the overlay of free Nurr1-LBD (red) and Nurr1-LBD with PGA1 (1:5, green; 1:10, blue), with residues showing chemical shift perturbations (with arrows) or intensity changes (boxed in red) labelled. Residues (Leu559, Gln571 and Thr595) showing additional peaks upon PGA1 incubation are marked with asterisks (*). This indicates that the PGE1 (a) and PGA1 (c) interaction with Nurr1-LBD matches the typical two-state binding model (P + L ⇄ PL) and an induced-fit binding model (P + L ⇄ PLopen → PLclosed), respectively. (d) Mapping of Nurr1-LBD residues perturbed in the presence of PGA1 reveals that both PGE1 (a and b) and PGA1 (c and d) recognize the same binding region on Nurr1-LBD, with maximum perturbation observed in helices H11 and H12. Residues showing chemical shifts and line broadening are coloured in purple while L410 is coloured in red (b and d), as its peak disappeared upon PGA1 binding. (e, f) PGA1 increases the transcriptional activity of Nurr1-based reporter constructs: Nurr1-LBD-dependent (e) and full-length Nurr1-dependent (f) transcriptional activities in SK-N-BE(2)C cells. n = 3 independent experiments, Data are presented as mean ± s.e.m.
Extended Data Fig. 3 Chemical shift perturbation plot of Nurr1-LBD upon PGE1 and PGA1 binding.
Chemical shift perturbation plot of Nurr1-LBD upon PGE1 (a) / PGA1 (b) binding (1:10 ratio) and their corresponding peak intensity plots (PGE1 (c) / PGA1 (d)) revealing residues with perturbed resonances and/or line broadening upon ligand binding. (*) denotes the peak belonging to L410 which disappeared upon PGA1 binding.
Extended Data Fig. 4 PGE1 conversion to PGA1 under crystal condition.
(a) The overlaid 2Fo-Fc (blue) and composite omit (pink) electron density maps contoured at 1σ cut-off confirming the conversion of PGE1 to PGA1, evident from the covalent bonding density with Cys566. (b) Mass spectrometry data of PGE1 incubated with Nurr1-LBD under crystallization buffer condition (100 mM MES, pH 5.5 and 200 mM MgCl2) confirming the conversion of PGE1 to PGA1, as revealed by the covalent complex molecular mass of 30,862 Da (Nurr1-LBD328–598 is 30,525 Da and PGA1 is 336.5 Da).
Extended Data Fig. 5 Crystal structure of PGA1-bound Nurr1-LBD and its molecular and functional analyses.
(a) Cartoon representation of Nurr1-LBD (blue) with PGA1 shown in sphere mode. (b) Interactions between PGA1 and Nurr1 residues (labelled) through hydrophobic contacts (grey broken lines) and hydrogen bonds (blue broken lines). Only chain B in the asymmetric unit are shown here, as the electron density for the PGA1 attached to this chain was complete. (c) PGJ2 and 15d-PGJ2 show no effect on the transcriptional activity of Nurr1-LBD. n = 3 independent experiments, Data are presented as mean ± s.e.m. (d) 15d-PGJ2 (3 μM), but not PGE1 (1 μM) or PGA1 (10 μM), induces the transcriptional activity of PPARγ-LBD.
Extended Data Fig. 6 Effects of mutations at Nurr1 residues interacting with the chain B (Arg515, His516, Arg563, Thr567).
(a), with the chain A (Phe443, Leu570, Ile573, Leu591) (b), and effects of mutations at the residue Cys566 (c) on PGA1 (10 μM)-induced transcriptional activation of Nurr1-LBD in SK-N-BE(2)C cells. n = 3 independent experiments, Data are presented as mean ± s.e.m.
Extended Data Fig. 7 Effects of EP2 agonists and antagonists on the transcriptional activity of Nurr1-LBD.
(a) The EP2 agonist, AH13205 activates Nurr1’s transcriptional activity, whereas EP3/EP4 agonists (Sulprostone and CAY10598) do not. (b) EP2 antagonist, PF-04418948 suppresses PGE1-induced transcriptional activation of Nurr1, whereas EP1/EP3/EP4 antagonists (SC-19220, L-798106, and L-161982) do not. (c) The synthetic PGE1 analogue misoprostol, activates Nurr1’s transcriptional activity in SK-N-BE(2)C cells. n = 3 independent experiments, Data are presented as mean ± s.e.m.
Extended Data Fig. 8 Protective effects of PGE1 and PGA1 against MPP+ in MN9D cells.
(a, b) Determination of protective effects of PGE1 and PGA1 in MN9D cells under MPP+-induced oxidative stress measured by MTT reduction. (a) Cells were treated with MPP+ (0–1000 µM) for 24 hrs. Cell viabilities assessed by MTT reduction assay show that treatment with 500 µM of MPP+ significantly induces 50% of cell death. (b) Pre-treatments with PGE1/PGA1 (24 hrs prior to MPP+ treatment) increase cell viability against the MPP+ induced oxidative stress in MN9D cells. *P < 0.05, **P < 0.01 compared to 0 µM; ###P < 0.001 compared to the absence of MPP+, unpaired two-tailed t-test; n = 3 independent samples per group. Data are mean ± s.e.m. (c, d) Protective effects of PGE1 and PGA1 measured by LDH release. (c) Cytotoxicity determined by LDH release assay also reveals that treatment with 500 µM of MPP+ significantly induces 50% of cell death in MN9D cells. (d) Similar to MTT reduction assay, pre-treatments with PGE1/PGA1 reduce cytotoxicity under the MPP+-induced oxidative stress. **P < 0.01, ***P < 0.001 compared to 0 µM; ###P < 0.001 compared to the absence of MPP+, unpaired two-tailed t-test; n = 3 independent samples per group. Data are mean ± s.e.m.
Extended Data Fig. 9 Effects of PGE1/PGA1 in the MPTP-induced reduction of DA levels.
The administration of PGE1/PGA1 significantly restores the MPTP-induced reduction of DA levels in the SN (a) and in the striatum (b). One-way ANOVA, Tukey’s post-hoc test; n = 5 per group. Data are mean ± s.e.m.
Extended Data Fig. 10 Mass spectrometry data between PGA1 and Nurr1-LBD under NMR condition.
Mass spectrometry data confirming the formation of the covalent bond between PGA1 (red line) with Nurr1-LBD356–598 (28.035 kDa), while PGE1 (blue dotted line) does not form such a covalent attachment under the NMR buffer conditions (20 mM sodium phosphate (pH 7.5) buffer containing 50 mM NaCl, 0.01% NaN3 in 90% H2O/10% D2O). The apo Nurr1-LBD356–598 (black line) (27.698 kDa) is shown for reference. The molecular weight of PGA1 is 336.5 Da. This also corroborates with the two-state binding and induced-fit model observed from NMR data (Extended Data Fig. 2a, c).
Supplementary information
Supplementary Information
Supplementary Figs. 1–18 and Tables 1–4.
Supplementary Video 1
PGA1 binding on Nurr1-LBD
Supplementary Video 2
Superposition of unbound over PGA1 bound Nurr1
Supplementary Video 3
Conformational changes induced by PGA1 binding
Source data
Source Data Fig. 1
Uncut Gel for Fig. 1g
Source Data Fig. 2
Uncut Gel for Fig. 2d
Rights and permissions
About this article
Cite this article
Rajan, S., Jang, Y., Kim, CH. et al. PGE1 and PGA1 bind to Nurr1 and activate its transcriptional function. Nat Chem Biol 16, 876–886 (2020). https://doi.org/10.1038/s41589-020-0553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0553-6
This article is cited by
-
An optimized Nurr1 agonist provides disease-modifying effects in Parkinson’s disease models
Nature Communications (2023)
-
Spotlight on pyroptosis: role in pathogenesis and therapeutic potential of ocular diseases
Journal of Neuroinflammation (2022)
-
A Nurr1 ligand C-DIM12 attenuates brain inflammation and improves functional recovery after intracerebral hemorrhage in mice
Scientific Reports (2022)
-
Prostaglandin A2 Interacts with Nurr1 and Ameliorates Behavioral Deficits in Parkinson’s Disease Fly Model
NeuroMolecular Medicine (2022)
-
Potent synthetic and endogenous ligands for the adopted orphan nuclear receptor Nurr1
Experimental & Molecular Medicine (2021)