Abstract
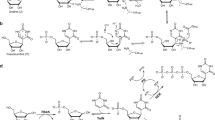
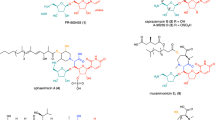
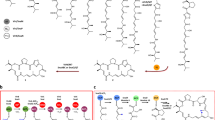
Several nucleoside antibiotics are structurally characterized by a 5″-amino-5″-deoxyribose (ADR) appended via a glycosidic bond to a high-carbon sugar nucleoside (5′S,6′S)-5′-C-glycyluridine (GlyU). GlyU is further modified with an N-alkylamine linker, the biosynthetic origin of which has yet to be established. By using a combination of feeding experiments with isotopically labeled precursors and characterization of recombinant proteins from multiple pathways, the biosynthetic mechanism for N-alkylamine installation for ADR–GlyU-containing nucleoside antibiotics has been uncovered. The data reveal S-adenosyl-l-methionine (AdoMet) as the direct precursor of the N-alkylamine, but, unlike conventional AdoMet- or decarboxylated AdoMet-dependent alkyltransferases, the reaction is catalyzed by a pyridoxal-5′-phosphate-dependent aminobutyryltransferase (ABTase) using a stepwise γ-replacement mechanism that couples γ-elimination of AdoMet with aza-γ-addition onto the disaccharide alkyl acceptor. In addition to using a conceptually different strategy for AdoMet-dependent alkylation, the newly discovered ABTases require a phosphorylated disaccharide alkyl acceptor, revealing a cryptic intermediate in the biosynthetic pathway.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Winn, M., Goss, R. J., Kimura, K. & Bugg, T. D. Antimicrobial nucleoside antibiotics targeting cell wall assembly: recent advances in structure–function studies and nucleoside biosynthesis. Nat. Prod. Rep. 27, 279–304 (2010).
Shiraishi, T. & Kuzuyama, T. Recent advances in the biosynthesis of nucleoside antibiotics. J. Antibiot. 72, 913–923 (2019).
Hirano, S., Ichikawa, S. & Matsuda, A. Total synthesis of (+)-FR-900493 and establishment of its absolute stereochemistry. Tetrahedron 63, 2798–2804 (2007).
Igarashi, M. et al. Caprazamycins, novel lipo-nucleoside antibiotics, from Streptomyces sp. II. Structure elucidation of caprazamycins. J. Antibiot. 58, 327–337 (2005).
Fujita, Y. et al. A-90289 A and B, new inhibitors of bacterial translocase I, produced by Streptomyces sp. SANK 60405. J. Antibiot. 64, 495–501 (2011).
Funabashi, M. et al. Structure-based gene targeting discovery of sphaerimicin, a bacterial translocase I inhibitor. Angew. Chem. Int. Ed. 52, 11607–11611 (2013).
McDonald, L. A. et al. Structures of the muraymycins, novel peptidoglycan biosynthesis inhibitors. J. Am. Chem. Soc. 124, 10260–10261 (2002).
Yang, Z. et al. Characterization of LipL as a non-heme, Fe(II)-dependent α-ketoglutarate:UMP dioxygenase that generates uridine-5′-aldehyde during A-90289 biosynthesis. J. Biol. Chem. 286, 7885–7892 (2011).
Barnard-Britson, S. et al. Amalgation of nucleosides and amino acids in antibiotic biosynthesis: discovery of an l-threonine:uridine-5′-aldehyde transaldolase. J. Am. Chem. Soc. 134, 18514–18517 (2012).
Chi, X., Pahari, P., Nonaka, K. & Van Lanen, S. G. Biosynthetic origin and mechanism of formation of the aminoribosyl moiety of peptidyl nucleoside antibiotics. J. Am. Chem. Soc. 133, 14452–14459 (2011).
Cui, Z. et al. Enzymatic synthesis of the ribosylated glycyl-uridine disaccharide core of peptidyl nucleoside antibiotics. J. Org. Chem. 83, 7239–7249 (2018).
Kaysser, L. et al. Identification and manipulation of the caprazamycin gene cluster lead to new simplified liponucleoside antibiotics and give insights into the biosynthetic pathway. J. Biol. Chem. 284, 14987–14996 (2009).
Michael, A. J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem. J. 473, 2315–2329 (2016).
Dreyfus, C., Lemaire, D., Mari, S., Pignol, D. & Arnoux, P. Crystallographic snapshots of iterative substrate translocations during nicotianamine synthesis in archaea. Proc. Natl. Acad. Sci. USA 106, 16180–16184 (2009).
Lee, J. et al. An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholera. J. Biol. Chem. 284, 9899–9907 (2009).
Cui, Z. et al. Self-resistance during muraymycin biosynthesis: a complementary nucleotidyltransferase and phosphotransferase with identical modification sites and distinct temporal order. Antimicrob. Agents Chemother. 62, e00193-18 (2018).
Cheng, L. et al. Identification of the gene cluster involved in muraymycin biosynthesis from Streptomyces sp. NRRL 30471. Mol. Biosyst. 7, 920–927 (2011).
Ikeguchi, Y., Bewley, M. C. & Pegg, A. E. Aminopropyltransferases: function, structure, and genetics. J. Biochem. 139, 1–9 (2006).
Martin, J. L. & McMillan, F. M. SAM (dependent) I AM: the S-adenosylmethioine-dependent methyltransferase fold. Curr. Opin. Struct. Biol. 12, 783–793 (2002).
Reeve, A. M., Breazeale, S. D. & Townsend, C. A. Purification, characterization, and cloning of an S-adenosylmethionine-dependent 3-amino-3-carboxypropyltransferase in nocardicin biosynthesis. J. Biol. Chem. 1998, 30695–30703 (1998).
Ramalingam, K., Lee, K.-M., Woodard, R. W., Bleecker, A. B. & Kende, H. Stereochemical course of the reaction catalyzed by the pyridoxal phosphate-dependent enzyme 1-aminocyclopropane-1-carboxylate synthase. Proc. Natl. Acad. Sci. USA 82, 7820–7824 (1985).
Xu, Z., Pan, G., Zhou, H. & Shen, B. Discovery and characterization of 1-aminocyclopropane-1-carboxylic acid synthase of bacterial origin. J. Am. Chem. Soc. 149, 16957–16961 (2018).
Funabashi, M. et al. The biosynthesis of liposidomycin-like A-90289 antibiotics featuring a new type of sulfotransferase. ChemBioChem 11, 184–190 (2010).
Ko, S., Eliot, A. C. & Kirsch, J. F. S-Methylmethionine is both a substrate and an activator of 1-aminocyclopropane-1-carboxylate synthase. Arch. Biochem. Biophys. 42, 85–90 (2004).
Johnston, M., Marcotte, P., Donovan, J. & Walsh, C. Mechanistic studies with vinylglycine and β-haoloaminobutyrates as substrates for cystathionine γ-synthase from Salmonella typhimurium. Biochemistry 18, 1729–1738 (1979).
Feng, L. & Kirsch, J. F. l-Vinylglycine is an alternative substrate as well as mechanism-based inhibitor of 1-aminocyclopropane-1-carboxylate synthase. Biochemistry 39, 2436–2444 (2000).
Capitani, G., Tschopp, M., Eliot, A. C., Kirsch, J. F. & Grütter, M. G. Structure of ACC synthase inactivated by the mechanism-based inhibitor l-vinylglycine. FEBS Lett. 579, 2458–2462 (2005).
Guggenheim, S. & Flavin, M. Cystathionine γ-synthase from Salmonella. Spectral changes in the presence of substrates. J. Biol. Chem. 246, 3562–3568 (1971).
Chang, M. N. T. & Walsh, C. T. Stereochemical analysis of γ-replacement and γ-elimination processes catalyzed by a pyridoxal phosphate enzyme. J. Am. Chem. Soc. 103, 4921–4927 (1981).
Guggenheim, S. & Flavin, M. Cystathionine γ-synthase. A pyridoxal enzyme catalyzing rapid exchange of β and α hydrogen atoms in amino acids. J. Biol. Chem. 244, 6217–6227 (1969).
Krog, J. S. et al. 3-(3-amino-3-carboxypropyl)-5,6-dihydrouridine is one of two novel post-transcriptional modifications in tRNALys(UUU) from Trypanosoma brucei. FEBS J. 278, 4782–4796 (2011).
Nishimura, S., Taya, Y., Kuchino, Y. & Oashi, Z. Enzymatic synthesis of 3-(3-amino-3-carboxypropyl)uridine in Escherichia coli phenylalanine transfer RNA: transfer of the 3-amino-acid-3-carboxypropyl group from S-adenosylmethionine. Biochem. Biophys. Res. Commun. 57, 702–708 (1974).
Ikegami, F. et al. Biosynthesis in vitro of 2-(3-amino-3-carboxypropyl)-isoxazolin-5-one, the neurotoxic amino acid in Lathyrus odoratus. Biol. Pharm. Bull. 16, 732–734 (1993).
Taya, Y., Tanaka, Y. & Nishimura, S. Cell-free biosynthesis of discadenine, a spore germination inhibitor of Dictyostelium discoideum. FEBS Lett. 89, 326–328 (1978).
Schneider, G., Käck, H. & Lindqvist, Y. The manifold of vitamin B6 dependent enzymes. Structure 8, R1–R6 (2000).
Brzović, P., Litzenberger Holbrook, E., Greene, R. C. & Dun, M. F. Reaction mechanism of Escherichia coli cystathionine γ-synthase: direct evidence for a pyridoxamine derivative of vinylglyoxylate as a key intermediate in pyridoxal phosphate dependent γ-elimination and γ-replacement reaction. Biochemistry 29, 442–451 (1990).
Yamagata, S. Roles of O-acetyl-l-homoserine sulfhydrylases in microorganisms. Biochimie 71, 1125–1143 (1989).
Esaki, N., Tanaka, H., Uemuru, S., Suzuki, T. & Soda, K. Catalytic action of l-methionine γ-lyase on selenomethionine and selenols. Biochemistry 18, 407–410 (1979).
Sato, D. & Nozaki, T. Methionine gamma-lyase: the unique reaction mechanism, physiological roles, and therapeutic applications against infectious diseases and cancers. IUBMB Life 61, 1019–1028 (2009).
Omura, H. et al. Purification, characterization and gene cloning of thermostable O-acetyl-l-homoserine sulfhydrylase forming γ-cyano-α-aminobutyric acid. J. Biosci. Bioeng. 96, 53–58 (2003).
Chiku, T. et al. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 (2009).
Faulkner, J. R. et al. On the sequence of bond formation in loline alkaloid biosynthesis. ChemBioChem 7, 1078–1088 (2006).
Enders, D., Wang, C. & Liebich, J. X. Organocatalytic aza-Michael additions. Chem. Eur. J. 15, 11058–11076 (2009).
Freedy, A. M. et al. Chemoselective installation of amine bonds on proteins through aza-Michael ligation. J. Am. Chem. Soc. 139, 18365–18375 (2017).
Xu, F., Wu, Q., Chen, X., Lin, X. & Wu, Q. A single lipase-catalysed one-pot protocol combining aminolysis resolution and aza-Michael addition: an easy and efficient way to synthesise β-amino acid esters. Eur. J. Org. Chem. 2015, 5393–5401 (2015).
Chisuga, T., Miyanaga, A., Kudo, F. & Eguchi, T. Structural analysis of the dual-function thioesterase SAV606 unravels the mechanism of Michael addition of glycine to an α,β-unsaturated thioester. J. Biol. Chem. 292, 10926–10937 (2017).
Kobylarz, M. J. et al. Deciphering the substrate specificity of SbnA, the enzyme catalyzing the first step in staphyloferrin B biosynthesis. Biochemistry 55, 927–939 (2016).
Brotzel, F. & Mayr, H. Nucleophilicities of amino acids and peptides. Org. Biomol. Chem. 5, 3814–3820 (2007).
Clayden, J., Greeves, N. & Warren, S. Organic Chemistry, 2nd edn (Oxford University Press, 2012).
Shiraishi, T., Hiro, N., Igarashi, M., Nishiyama, M. & Kuzuyama, T. Biosynthesis of the antituberculosis agent caprazamycin: identification of caprazol-3″-phosphate, an unprecedented caprazamycin-related metabolite. J. Gen. Appl. Microbiol. 62, 164–166 (2016).
Spork, A. P., Koppermann, S., Dittrich, B., Herbst-Irmer, R. & Ducho, C. Efficient synthesis of the core structure of muraymycin and caprazamycin nucleoside antibiotics based on a stereochemically revised sulfur ylide reaction. Tetrahedron Asymmetry 21, 763–766 (2010).
Hirano, S., Ichikawa, S. & Matsuda, A. Total synthesis of caprazol, a core structure of the caprazamycin antituberculosis antibiotics. Angew. Chem. Int. Ed. 44, 1854–1856 (2005).
Cohen, S. A. & Michaud, D. P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 211, 279–287 (1993).
Zhang, J. & Klinman, J. P. High-performance liquid chromatography separation of the (S,S)- and (R,S)-forms of S-adenosyl-l-methionine. Anal. Biochem. 476, 81–83 (2015).
Tanaka, H., Yamamoto, A., Ishida, T. & Horiike, K. Simultaneous measurement of d-serine dehydratase and d-amino acid oxidase activities by the detection 2-oxo-acid formation with reverse-phase high performance liquid chromatography. Anal. Biochem. 362, 83–88 (2007).
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH, grant nos. AI087849 and GM115261), the National Center for Advancing Translational Sciences (grant nos. UL1TR000117 and UL1TR001998), the Deutsche Forschungsgemeinschaft (grant no. DU1095/5-1), the state of Lower Saxony (Lichtenberg doctoral fellowship (CaSuS program) for A.L.), the Konrad-Adenauer-Stiftung (doctoral fellowship for D.W.) and the Fonds der Chemischen Industrie (doctoral fellowship for G.N.). NMR (600 MHz) data were collected at the NMR facility of the Center for Environmental Systems Biochemistry supported in part by the NIH (grant no. DK097215). We thank the Department of Pharmaceutical Sciences and the College of Pharmacy for financial support for MS and NMR instrumentation.
Author information
Authors and Affiliations
Contributions
Z.C. and J.O. performed and analyzed the data for cloning, heterologous expression, and protein functional assignments and biochemical characterization. Z.C., X.W. and M.B. performed MS and NMR spectroscopy of enzymatic products and analyzed the respective data. X.L, Y.Z., A.L., D.W. and G.N. performed the chemical synthesis and analyzed the respective data. Z.C., J.S.T., C.D. and S.G.v.L. conceived the study, directed and planned the research, analyzed the data and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1–23
Rights and permissions
About this article
Cite this article
Cui, Z., Overbay, J., Wang, X. et al. Pyridoxal-5′-phosphate-dependent alkyl transfer in nucleoside antibiotic biosynthesis. Nat Chem Biol 16, 904–911 (2020). https://doi.org/10.1038/s41589-020-0548-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0548-3
This article is cited by
-
A pyridoxal 5′-phosphate-dependent Mannich cyclase
Nature Catalysis (2023)
-
β-Hydroxylation of α-amino-β-hydroxylbutanoyl-glycyluridine catalyzed by a nonheme hydroxylase ensures the maturation of caprazamycin
Communications Chemistry (2022)
-
β-NAD as a building block in natural product biosynthesis
Nature (2021)
-
Cryptic phosphorylation in nucleoside natural product biosynthesis
Nature Chemical Biology (2021)