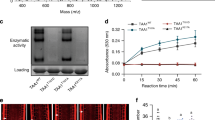
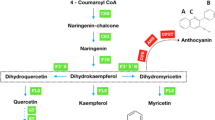
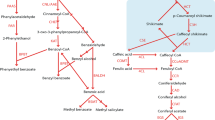
Abstract
In plants, phenylalanine biosynthesis occurs via two compartmentally separated pathways. Overexpression of petunia chorismate mutase 2 (PhCM2), which catalyzes the committed step of the cytosolic pathway, increased flux in cytosolic phenylalanine biosynthesis, but paradoxically decreased the overall levels of phenylalanine and phenylalanine-derived volatiles. Concomitantly, the levels of auxins, including indole-3-acetic acid and its precursor indole-3-pyruvic acid, were elevated. Biochemical and genetic analyses revealed the existence of metabolic crosstalk between the cytosolic phenylalanine biosynthesis and tryptophan-dependent auxin biosynthesis mediated by an aminotransferase that uses a cytosolic phenylalanine biosynthetic pathway intermediate, phenylpyruvate, as an amino acceptor for auxin formation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All figures except Fig. 4 have associated raw data available from the corresponding author upon reasonable request. Plant material generated in this study is available from the corresponding author upon request. The PhTrp-AT sequence reported in this paper has been deposited in GenBank database under accession number MN233649.
References
Maeda, H. & Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 63, 73–105 (2012).
Qian, Y. et al. Completion of the cytosolic post-chorismate phenylalanine biosynthetic pathway in plants. Nat. Commun. 10, 15 (2019).
Westfall, C. S., Xu, A. & Jez, J. M. Structural evolution of differential amino acid effector regulation in plant chorismate mutases. J. Biol. Chem. 289, 28619–28628 (2014).
Tzin, V., Malitsky, S., Aharoni, A. & Galili, G. Expression of a bacterial bi-functional chorismate mutase/prephenate dehydratase modulates primary and secondary metabolism associated with aromatic amino acids in Arabidopsis. Plant J. 60, 156–167 (2009).
Verdonk, J. C. et al. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62, 997–1008 (2003).
Boatright, J. et al. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 135, 1993–2011 (2004).
Orlova, I. et al. Reduction of benzenoid synthesis in petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport. Plant Cell 18, 3458–3475 (2006).
Yoo, H. et al. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine:phenylpyruvate aminotransferase. Nat. Commun. 4, 2833 (2013).
Widhalm, J. R. et al. Identification of a plastidial phenylalanine exporter that influences flux distribution through the phenylalanine biosynthetic network. Nat. Commun. 6, 8142 (2015).
Maeda, H. et al. RNAi suppression of Arogenate dehydratase1 reveals that phenylalanine is synthesized predominantly via the arogenate pathway in petunia petals. Plant Cell 22, 832–849 (2010).
Corea, O. R. A. et al. Arogenate dehydratase isoenzymes profoundly and differentially modulate carbon flux into lignins. J. Biol. Chem. 287, 11446–11459 (2012).
Dal Cin, V. et al. Identification of genes in the phenylalanine metabolic pathway by ectopic expression of a MYB transcription factor in tomato fruit. Plant Cell 23, 2738–2753 (2011).
Pyke, K. A. & Page, A. M. Plastid ontogeny during petal development in Arabidopsis. Plant Physiol. 116, 797–803 (1998).
Marano, M. R., Serra, E. C., Orellano, E. G. & Carrillo, N. The path of chromoplast development in fruits and flowers. Plant Sci. 94, 1–17 (1993).
Kobayashi, K. et al. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell 24, 1081–1095 (2012).
Miyazawa, Y. et al. Auxin and cytokinin have opposite effects on amyloplast development and the expression of starch synthesis genes in cultured Bright Yellow-2 tobacco cells. Plant Physiol. 121, 461–470 (1999).
Mashiguchi, K. et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 18512–18517 (2011).
Zhao, Y. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 5, 334–338 (2012).
Tao, Y. et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176 (2008).
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008).
Schneider, P., Weber, M., Rosenberger, K. & Hoffmeister, D. A one-pot chemoenzymatic synthesis for the universal precursor of antidiabetes and antiviral bis-indolylquinones. Chem. Biol. 14, 635–644 (2007).
Bombarely, A. et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants 2, 16074 (2016).
Aloni, R., Aloni, E., Langhans, M. & Ullrich, C. I. Role of auxin in regulating Arabidopsis flower development. Planta 223, 315–328 (2006).
Colquhoun, T. A. et al. Petunia floral volatile benzenoid/phenylpropanoid genes are regulated in a similar manner. Phytochemistry 71, 158–167 (2010).
He, W. et al. A small-molecule screen identifies l-kynurenine as a competitive inhibitor of TAA1/TAR Activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23, 3944–3960 (2011).
Zheng, Z. et al. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat. Chem. Biol. 9, 244–246 (2013).
Krizek, B. A. Auxin regulation of Arabidopsis flower development involves members of the AINTEGUMENTA-LIKE/PLETHORA (AIL/PLT) family. J. Exp. Bot. 62, 3311–3319 (2011).
Fürtauer, L., Küstner, L., Weckwerth, W., Heyer, A. G. & Nägele, T. Resolving subcellular plant metabolism. Plant J. 100, 438–455 (2019).
Hentrich, M. et al. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. 74, 626–637 (2013).
Korver, R. A., Koevoets, I. T. & Testerink, C. Out of shape during stress: a key role for auxin. Trends Plant Sci. 23, 783–793 (2018).
Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 3, 2–20 (2010).
Gleave, A. P. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20, 1203–1207 (1992).
Karimi, M., Inzé, D. & Depicker, A. GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002).
Klempien, A. et al. Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell 24, 2015–2030 (2012).
Lu, Q., Chen, L., Lu, M., Chen, G. & Zhang, L. Extraction and analysis of auxins in plants using dispersive liquid−liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 58, 2763–2770 (2010).
Ahmad, S. & Jensen, R. A. A simple spectrophotometric assay for arogenate dehydratase. Anal. Biochem. 163, 107–111 (1987).
Smith, A. M. & Zeeman, S. C. Quantification of starch in plant tissues. Nat. Protoc. 1, 1342–1345 (2006).
Nelson, B. K., Cai, X. & Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136 (2007).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Acknowledgements
This work was supported by grant MCB-1519083 from the US National Science Foundation to J.A.M. and N.D., by the USDA National Institute of Food and Agriculture Hatch project 177845 to N.D., and by the USDA National Institute of Food and Agriculture postdoctoral grant 2016-67012-24699 to J.H.L. The authors thank A. Withrow (Michigan State University) for assisting with transmission electron microscopy and B. Dilkes for discussion of Arabidopsis experiments with gravitropism.
Author information
Authors and Affiliations
Contributions
J.H.L., Y.Q., and N.D. designed research; Y.Q. generated PhCM2- and AtVAS-overexpression transgenic plants, and performed their metabolic profiling, analysis of chorismate mutase activity, and stable isotope labeling experiments. J.H.L. performed starch and auxin profiling of petunia transgenic plants and Arabidopsis cm2 mutants, feeding experiments with IAA and Trp-AT amino acceptors, and biochemical characterization of PhTrp-AT, and prepared samples for transmission electron microscopy. A.G. performed starch and auxin profiling in petunia petals. L.G. performed metabolic flux modeling. I.M. analyzed PhTrp-AT expression. X.-Q.H. analyzed PhTrp-At subcellular localization. G.L. and M.E.B. contributed to biochemical characterization of aminotransferase. Y.Q., J.H.L., L.G., I.M., X-Q.H., G.L., M.E.B., J.P.N., J.A.M., and N.D. analyzed data. J.H.L. and N.D. wrote the paper. All authors read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs 1–11 and Tables 1–6
Rights and permissions
About this article
Cite this article
Lynch, J.H., Qian, Y., Guo, L. et al. Modulation of auxin formation by the cytosolic phenylalanine biosynthetic pathway. Nat Chem Biol 16, 850–856 (2020). https://doi.org/10.1038/s41589-020-0519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0519-8
This article is cited by
-
An ARF gene mutation creates flint kernel architecture in dent maize
Nature Communications (2024)
-
Phenylalanine affects betalain biosynthesis and promotes ascorbic acid, α-tocopherol, and retinol accumulation in Amaranthus tricolor seedlings
Acta Physiologiae Plantarum (2024)
-
Multi-omics quantitative data of tomato fruit unveils regulation modes of least variable metabolites
BMC Plant Biology (2023)
-
Carbon and nitrogen metabolism affects kentucky bluegrass rhizome expansion
BMC Plant Biology (2023)
-
Cuticle thickness affects dynamics of volatile emission from petunia flowers
Nature Chemical Biology (2021)