Abstract
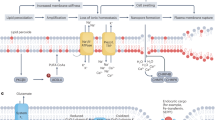
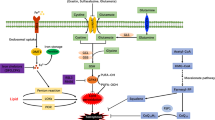
Ferroptotic death is the penalty for losing control over three processes—iron metabolism, lipid peroxidation and thiol regulation—that are common in the pro-inflammatory environment where professional phagocytes fulfill their functions and yet survive. We hypothesized that redox reprogramming of 15-lipoxygenase (15-LOX) during the generation of pro-ferroptotic signal 15-hydroperoxy-eicosa-tetra-enoyl-phosphatidylethanolamine (15-HpETE-PE) modulates ferroptotic endurance. Here, we have discovered that inducible nitric oxide synthase (iNOS)/NO•-enrichment of activated M1 (but not alternatively activated M2) macrophages/microglia modulates susceptibility to ferroptosis. Genetic or pharmacologic depletion/inactivation of iNOS confers sensitivity on M1 cells, whereas NO• donors empower resistance of M2 cells to ferroptosis. In vivo, M1 phagocytes, in comparison to M2 phagocytes, exert higher resistance to pharmacologically induced ferroptosis. This resistance is diminished in iNOS-deficient cells in the pro-inflammatory conditions of brain trauma or the tumour microenvironment. The nitroxygenation of eicosatetraenoyl (ETE)-PE intermediates and oxidatively truncated species by NO• donors and/or suppression of NO• production by iNOS inhibitors represent a novel redox mechanism of regulation of ferroptosis in pro-inflammatory conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw data are available at the following link https://data.mendeley.com/datasets/t4jyhhf3gw/draft?a=76c6ac56-4305-463e-bebc-b004c2000edb.
Code used for the analysis of the MD simulations of NO• interactions with 15-LOX has been made available in two formats: (1) Jupyter Notebook and (2) html (https://onedrive.live.com/?authkey=%21AFEVmP5sOP1km0s&id=4960CA1B5C7F3FD%219568&cid=04960CA1B5C7F3FD).
References
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Stoyanovsky, D. A. et al. Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 133, 153–161 (2018).
Gugiu, B. G. et al. Identification of oxidatively truncated ethanolamine phospholipids in retina and their generation from polyunsaturated phosphatidylethanolamines. Chem. Res. Toxicol. 19, 262–271 (2006).
Pizzimenti, S. et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 4, 242 (2013).
Hoff, H. F., O’Neil, J., Wu, Z., Hoppe, G. & Salomon, R. L. Phospholipid hydroxyalkenals: biological and chemical properties of specific oxidized lipids present in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 23, 275–282 (2003).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Wenzel, S. E. et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171, 628–641 (2017).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Conrad, M. et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 32, 602–619 (2018).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Feng, H. & Stockwell, B. R. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 16, e2006203 (2018).
Murray, P. J. Macrophage polarization. Annu. Rev. Physiol. 79, 541–566 (2017).
O’Donnell, V. B. et al. 15-Lipoxygenase catalytically consumes nitric oxide and impairs activation of guanylate cyclase. J. Biol. Chem. 274, 20083–20091 (1999).
Lorsbach, R. B., Murphy, W. J., Lowenstein, C. J., Snyder, S. H. & Russell, S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J. Biol. Chem. 268, 1908–1913 (1993).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Corna, G. et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 95, 1814–1822 (2010).
Zhang, Y. et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem. Biol. 26, 623–633 (2019).
Borland, C. et al. Permeability and diffusivity of nitric oxide in human plasma and red cells. Nitric Oxide 78, 51–59 (2018).
Dar, H. H. et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Invest. 128, 4639–4653 (2018).
Yamanaka, K. et al. A novel fluorescent probe with high sensitivity and selective detection of lipid hydroperoxides in cells. RSC Adv. 2, 7894–7900 (2012).
Shah, R., Shchepinov, M. S. & Pratt, D. A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 4, 387–396 (2018).
Monroe, L. L. et al. Zymosan-induced peritonitis: effects on cardiac function, temperature regulation, translocation of bacteria, and role of dectin-1. Shock 46, 723–730 (2016).
Kenny, E. M. et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit. Care Med. 47, 410–418 (2018).
Bayir, H. et al. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 25, 673–684 (2005).
Loane, D. J. & Kumar, A. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp. Neurol. 275, 316–327 (2016).
Bayir, H. et al. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J. Neurochem. 101, 168–181 (2007).
Foley, L. M. et al. Magnetic resonance imaging assessment of macrophage accumulation in mouse brain after experimental traumatic brain injury. J. Neurotrauma 26, 1509–1519 (2009).
Brune, B. et al. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 19, 595–637 (2013).
Ivanov, I., Kuhn, H. & Heydeck, D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 573, 1–32 (2015).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Kanazawa, M., Ninomiya, I., Hatakeyama, M., Takahashi, T. & Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int. J. Mol. Sci. 18, 2135 (2017).
Anthonymuthu, T. S. et al. Empowerment of 15-lipoxygenase catalytic competence in selective oxidation of membrane ETE-PE to ferroptotic death signals, HpETE-PE. J. Am. Chem. Soc. 140, 17835–17839 (2018).
Zilka, O. et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent. Sci. 3, 232–243 (2017).
Cao, J. Y. & Dixon, S. J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–2209 (2016).
Rubbo, H. et al. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch. Biochem. Biophys. 324, 15–25 (1995).
Saam, J., Ivanov, I., Walther, M., Holzhutter, H. G. & Kuhn, H. Molecular dioxygen enters the active site of 12/15-lipoxygenase via dynamic oxygen access channels. Proc. Natl Acad. Sci. USA 104, 13319–13324 (2007).
Rubbo, H. et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 269, 26066–26075 (1994).
O’Donnell, V. B. et al. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with ɑ-tocopherol. Biochemistry 36, 15216–15223 (1997).
Napoli, C. et al. Effects of nitric oxide on cell proliferation: novel insights. J. Am. Coll. Cardiol. 62, 89–95 (2013).
Thomas, D. D. et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 45, 18–31 (2008).
Perrotta, C. et al. Nitric oxide generated by tumor-associated macrophages is responsible for cancer resistance to cisplatin and correlated with syntaxin 4 and acid sphingomyelinase inhibition. Front. Immunol. 9, 1186 (2018).
Xie, B. S. et al. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 25, 465–475 (2019).
Amaral, E. P. et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 216, 556–570 (2019).
Martin-Sanchez, D. et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 28, 218–229 (2017).
Uderhardt, S. et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity 36, 834–846 (2012).
Wu, Y. L. et al. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc. Natl Acad. Sci. USA 103, 1852–1857 (2006).
RayA. & DittelB. N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 35, e1488 (2010).
Lian, H., Roy, E. & Zheng, H. Protocol for primary microglial culture preparation. Bio Protoc 6, e1989 (2016).
Elmore, M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014).
Weischenfeldt, J. & Porse, B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008, pdb.prot5080 (2008).
Harris, L. A. et al. BioNetGen 2.2: advances in rule-based modeling. Bioinformatics 32, 3366–3368 (2016).
Liu, B., Gyori, B. & Thiagarajan, P. In Automated Reasoning for Systems Biology and Medicine (eds. Liò, P. & Zuliani, P.) 63–92 (Springer International Publishing, Cham; 2019).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Tovchigrechko, A. & Vakser, I. A. GRAMM-X public web server for protein–protein docking. Nucleic Acids Res. 34, W310–W314 (2006).
Frisch, M. et al. Gaussian 03, Revision B.05 (Gaussian, 2003).
Bakan, A. et al. Evol and ProDy for bridging protein sequence evolution and structural dynamics. Bioinformatics 30, 2681–2683 (2014).
Acknowledgements
This work was supported by NIH (HL114453-06, U19AI068021, CA165065-06, NS076511, NS061817, P41GM103712) and by Russian academic excellence project ‘5-100’.
Author information
Authors and Affiliations
Contributions
V.E.K. and H.B. conceived the study. A.A.K., Q.Y., H.H.D., G.V.S., H.-C.T., M.A.A. and L.A.P. performed experiments with cells. V.E.K., H.B. and D.I.G. designed in vivo experiments. Q.Y., Y.L.W., R.K. and Y.G. performed in vivo experiments. A.A.K., Q.Y. and H.H.D. analyzed the data. Y.Y.T., T.S.A. and V.A.T. performed MS measurements and analyzed data. Y.Y.T., T.S.A. and R.M.D. discussed and interpreted MS results. K.M.-R., B.L. and I.H.S. performed computational modelling. I.B. supervised computational studies. C.M.S.C. performed imaging experiments and participated in interpreting them. H.B., D.A.S., R.K.M. and D.I.G. participated in formulating the idea and interpreting the data. P.S.T. and J.S.G. participated in the discussion and helped in writing the manuscript. Y.Y.T., I.B. and H.B. participated in writing the manuscript. H.B. and V.E.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11.
Supplementary Tables 1 and 2
Supplementary Tables 1 and 2.
Supplementary Video 1
Fragment of MD simulations of competitive binding of NO and O2 molecules to 15-LOX-2.
Rights and permissions
About this article
Cite this article
Kapralov, A.A., Yang, Q., Dar, H.H. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol 16, 278–290 (2020). https://doi.org/10.1038/s41589-019-0462-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0462-8
This article is cited by
-
Modulating ferroptosis sensitivity: environmental and cellular targets within the tumor microenvironment
Journal of Experimental & Clinical Cancer Research (2024)
-
Targeting ferroptosis for leukemia therapy: exploring novel strategies from its mechanisms and role in leukemia based on nanotechnology
European Journal of Medical Research (2024)
-
Melatonin: a ferroptosis inhibitor with potential therapeutic efficacy for the post-COVID-19 trajectory of accelerated brain aging and neurodegeneration
Molecular Neurodegeneration (2024)
-
Disease and brain region specific immune response profiles in neurodegenerative diseases with pure and mixed protein pathologies
Acta Neuropathologica Communications (2024)
-
Harnessing ferroptosis for enhanced sarcoma treatment: mechanisms, progress and prospects
Experimental Hematology & Oncology (2024)