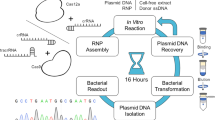
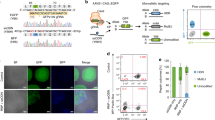
Abstract
Here, we report a rapid CRISPR–Cas9-mediated gene knock-in strategy that uses Cas9 ribonucleoprotein and 5′-modified double-stranded DNA donors with 50-base-pair homology arms and achieved unprecedented 65/40% knock-in rates for 0.7/2.5 kilobase inserts, respectively, in human embryonic kidney 293T cells. The identified 5′-end modification led to up to a fivefold increase in gene knock-in rates at various genomic loci in human cancer and stem cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Change history
15 January 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41589-020-0470-8
References
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Doudna, J. A. et al. The new frontier of genome engineering with CRISPR–Cas9. Science 346, 1258096 (2014).
Leonetti, M. D. et al. A scalable strategy for high-throughput GFP tagging of endogenous human proteins. Proc. Natl Acad. Sci. USA 113, 3501–3508 (2016).
Diao, Y. R. et al. A tiling-deletion-based genetic screen for cis-regulatory element identification in mammalian cells. Nat. Methods 14, 629–633 (2017).
Stephens, C. J. et al. Targeted in vivo knock-in of human alpha-1-antitrypsin cDNA using adenoviral delivery of CRISPR/Cas9. Gene Ther. 25, 139–156 (2018).
Merkle, F. T. et al. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell Rep. 11, 875–883 (2015).
Renaud, J. B. et al. Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR-Cas9 nucleases. Cell Rep. 14, 2263–2272 (2016).
He, X. et al. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 44, e85 (2016).
Paix, A. et al. Precision genome editing using synthesis-dependent repair of Cas9-induced DNA breaks. Proc. Natl Acad. Sci. USA 114, 10745–10754 (2017).
Tasan, I. et al. CRISPR/Cas9-mediated knock-in of an optimized TetO repeat for live cell imaging of endogenous loci. Nucleic Acids Res. 46, e100 (2018).
Brinkman, E. K. et al. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Gutierrez-Triana, J. A. et al. Efficient single-copy HDR by 5′ modified long dsDNA donors. eLife 7, e39468 (2018).
DeWitt, M. A. et al. Genome editing via delivery of Cas9 ribonucleoprotein. Methods 121-122, 9–15 (2017).
Hur, J. K. et al. Targeted mutagenesis in mice by electroporation of Cpf1 ribonucleoproteins. Nat. Biotechnol. 34, 807–808 (2016).
Strukov Y. G. et al. Development of mammalian cell lines with lac operator-tagged chromosomes. Cold Spring Harbor Protoc. https://doi.org/10.1101/pdb.prot4903 (2008).
Hindson, B. J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604–8610 (2011).
Acknowledgements
This work was supported by the US National Institutes of Health (grant nos. 1UM1HG009402 and 1U54DK107965) (H.Z.). We thank B. Pilas and B. Balhan (Flow Cytometry Facility, Biotechnology Center, University of Illinois at Urbana Champaign) for cell sorting experiments. We thank S. Long and K. Sobanska for help with ddPCR experiments. We thank W. Tang, T. Si, J. Lian, C. Field and S. Tsai for helpful suggestions for manuscript writing and experimental designs.
Author information
Authors and Affiliations
Contributions
Y.Y and H.Z. designed and conceived the study. Y.Y., Y.G. and Q.T. performed most of the experiments and analyzed the data. Y.L. and H.Y. contributed to construct generation, dsDNA donor preparation and fluorescence activated cell sorting analysis. M.Z. contributed to single-guide RNA preparation and gene KI experiments in HEK293T cell lines. I.T. contributed to gene KI experiments in the H1 and WTC G3 cell lines. S.J. contributed to gene KI experiments in the H1 cell line and off-target analysis. H.Z. supervised the research. Y.Y., Y.G., Q.T. and H.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and Figs. 1–6.
Rights and permissions
About this article
Cite this article
Yu, Y., Guo, Y., Tian, Q. et al. An efficient gene knock-in strategy using 5′-modified double-stranded DNA donors with short homology arms. Nat Chem Biol 16, 387–390 (2020). https://doi.org/10.1038/s41589-019-0432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0432-1
This article is cited by
-
Optimal tagging strategies for illuminating expression profiles of genes with different abundance in zebrafish
Communications Biology (2023)
-
SHIELD: a platform for high-throughput screening of barrier-type DNA elements in human cells
Nature Communications (2023)
-
Application of combinatorial optimization strategies in synthetic biology
Nature Communications (2020)