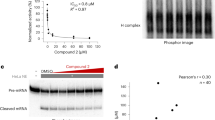
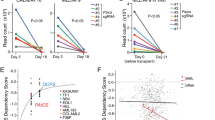
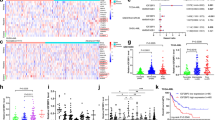
Abstract
The post-genomic era has seen many advances inBaryza and G. Rice for helpful our understanding of cancer pathways, yet resistance and tumor heterogeneity necessitate multiple approaches to target even monogenic tumors. Here, we combine phenotypic screening with chemical genetics to identify pre-messenger RNA endonuclease cleavage and polyadenylation specificity factor 3 (CPSF3) as the target of JTE-607, a small molecule with previously unknown target. We show that CPSF3 represents a synthetic lethal node in a subset of acute myeloid leukemia (AML) and Ewing’s sarcoma cancer cell lines. Inhibition of CPSF3 by JTE-607 alters expression of known downstream effectors in AML and Ewing’s sarcoma lines, upregulates apoptosis and causes tumor-selective stasis in mouse xenografts. Mechanistically, it prevents the release of newly synthesized pre-mRNAs, resulting in read-through transcription and the formation of DNA-RNA hybrid R-loop structures. This study implicates pre-mRNA processing, and specifically CPSF3, as a druggable target providing an avenue to therapeutic intervention in cancer.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are included in this published article (and its Supplementary Information files). The raw RNA-sequencing reads are available in the NCBI Sequence Read Archive under accession number SRP158650. X-ray structure data have been deposited to the PDB with the code 6M8Q. All other relevant data are available from the corresponding author on reasonable request.
Change history
02 April 2020
The article was updated on 04 March 2020.
04 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41589-020-0508-y
References
Swinney, D. C. & Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 10, 507–519 (2011).
Moffat, J. G., Vincent, F., Lee, J. A., Eder, J. & Prunotto, M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat. Rev. Drug Discov. 16, 531–543 (2017).
Schreiber, S. L. Chemical genetics resulting from a passion for synthetic organic chemistry. Bioorg. Med. Chem. 6, 1127–1152 (1998).
Carson, C. et al. Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS ONE 10, e0127498 (2015).
Rothman, D. M. et al. Metabolic enzyme sulfotransferase 1A1 is the trigger for N-benzyl indole carbinol tumor growth suppression. Chem. Biol. 22, 1228–1237 (2015).
Kakutani, M., Takeuchi, K., Waga, I., Iwamura, H. & Wakitani, K. JTE-607, a novel inflammatory cytokine synthesis inhibitor without immunosuppression, protects from endotoxin shock in mice. Inflamm. Res. 48, 461–468 (1999).
Borozdenkova, S. et al. Effects of a cytokine inhibitor, JTE-607, on the response to endotoxin in healthy human volunteers. Int. Immunopharmacol. 11, 1837–1843 (2011).
Ryugo, M. et al. Pharmacologic preconditioning of JTE-607, a novel cytokine inhibitor, attenuates ischemia-reperfusion injury in the myocardium. J. Thorac. Cardiovasc. Surg. 127, 1723–1727 (2004).
Jian, M. Y., Koizumi, T., Tsushima, K. & Kubo, K. JTE-607, a cytokine release blocker, attenuates acid aspiration-induced lung injury in rats. Eur. J. Pharmacol. 488, 231–238 (2004).
Uesato, N., Fukui, K., Maruhashi, J., Tojo, A. & Tajima, N. JTE-607, a multiple cytokine production inhibitor, ameliorates disease in a SCID mouse xenograft acute myeloid leukemia model. Exp. Hematol. 34, 1385–1392 (2006).
Tajima, N. et al. JTE-607, a multiple cytokine production inhibitor, induces apoptosis accompanied by an increase in p21waf1/cip1 in acute myelogenous leukemia cells. Cancer Sci. 101, 774–781 (2010).
Li, B. E. & Ernst, P. Two decades of leukemia oncoprotein epistasis: the MLL1 paradigm for epigenetic deregulation in leukemia. Exp. Hematol. 42, 995–1012 (2014).
Lessnick, S. L. & Ladanyi, M. Molecular pathogenesis of Ewing sarcoma: new therapeutic and transcriptional targets. Annu. Rev. Pathol. 7, 145–159 (2012).
Kim, N. & Jinks-Robertson, S. Transcription as a source of genome instability. Nat. Rev. Genet. 13, 204–214 (2012).
Danckwardt, S., Hentze, M. W. & Kulozik, A. E. 3′ end mRNA processing: molecular mechanisms and implications for health and disease. EMBO J. 27, 482–498 (2008).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Somervaille, T. C. P. et al. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell 4, 129–140 (2009).
Zuber, J. et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 25, 1628–1640 (2011).
Smith, R. et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing’s sarcoma. Cancer Cell 9, 405–416 (2006).
Tirode, F. et al. Mesenchymal stem cell features of Ewing tumors. Cancer Cell 11, 421–429 (2007).
Schirle, M. & Jenkins, J. L. Identifying compound efficacy targets in phenotypic drug discovery. Drug Discov. Today 21, 82–89 (2016).
MacKeigan, J. P., Murphy, L. O. & Blenis, J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat. Cell Biol. 7, 591–600 (2005).
König, R. et al. A probability-based approach for the analysis of large-scale RNAi screens. Nat. Methods 4, 847–849 (2007).
Zuberi, K. et al. GeneMANIA prediction server 2013 update. Nucleic Acids Res. 41, W115–W122 (2013).
Gower, C. M. et al. Conversion of a single polypharmacological agent into selective bivalent inhibitors of intracellular kinase activity. ACS Chem. Biol. 11, 121–131 (2016).
Thomas, J. R. et al. in Proteomics for Drug Discovery: Methods and Protocols (eds. Lazar, I. M. et al.) 1–18 (Springer, 2017).
Salcius, M. et al. SEC–TID: a label-free method for small-molecule target identification. J. Biomol. Screen. 19, 917–927 (2014).
Mandel, C. R. et al. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 444, 953–956 (2006).
Hill, C. H. et al. Activation of the endonuclease that defines mRNA 3′ ends requires incorporation into an 8-subunit core cleavage and polyadenylation factor complex. Mol. Cell 73, 1217–1231 (2019).
Bill, A. et al. Variomics screen identifies the re-entrant loop of the calcium-activated chloride channel ANO1 that facilitates channel activation. J. Biol. Chem. 290, 889–903 (2015).
Huang, Z. et al. A functional variomics tool for discovering drug-resistance genes and drug targets. Cell Rep. 3, 577–585 (2013).
Grohar, P. J. et al. Functional genomic screening reveals splicing of the EWS-FLI1 fusion transcript as a vulnerability in Ewing sarcoma. Cell Rep. 14, 598–610 (2016).
Riggi, N. et al. EWS-FLI1 utilizes divergent chromatin remodeling mechansims to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell 26, 668–681 (2014).
Tomazou, E. M. et al. Epigenome mapping reveals distinct modes of gene regulation and widespread enhancer reprogramming by the oncogenic fusion proteins EWS-FLI1. Cell Rep. 10, 1082–1095 (2015).
Zhao, L. et al. Integrated genome-wide chromatin occupancy and expression analyses identify key myeloid pro-differentiation transcription factors repressed by Myb. Nucleic Acids Res. 39, 4664–4679 (2011).
Kerry, J. et al. MLL-AF4 spreading identifies binding sites that are distinct from super-enhancers and that govern sensitivity to DOT1L inhibition in leukemia. Cell Rep. 18, 482–495 (2017).
Gorthi, A. et al. EWS-FLI1 increases transcription to cause R-Loops and block BRCA1 repair in Ewing sarcoma. Nature 555, 387–391 (2018).
Stirling, P. C. et al. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 26, 163–175 (2012).
Kakegawa, J., Sakane, N., Suzuki, K. & Yoshida, T. JTE-607, a multiple cytokine production inhibitor, targets CPSF3 and inhibits pre-mRNA processing. Biochem. Biophys. Res. Commun. 518, 32–37 (2019).
Iniguez, A. B. et al. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. Cancer Cell 33, 202–216 (2018).
Fidaleo, M. et al. Genotoxic stress inhibits Ewing sarcoma cell growth by modulating alternative pre-mRNA processing of the RNA helicase DHX9. Oncotarget 6, 31740–31757 (2015).
Selvanathan, S. P. et al. Oncogenic fusion protein EWS-FLI1 is a network hub that regulates alternative splicing. Proc. Natl Acad. Sci. USA 112, E1307–E1316 (2015).
Garnett, M. J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Dubbury, S. J., Boutz, P. L. & Sharp, P. A. CDK12 regulates DNA repair genes by suppressing intronic polyadenylation. Nature 564, 141–145 (2018).
Teloni, F. et al. Efficient pre-mRNA cleavage prevents replication-stress-associated genome instability. Mol. Cell 73, 670–683 (2019).
Kaida, D. et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 3, 576–583 (2007).
Palacino, J. et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 11, 511–517 (2015).
Sonoiki, E. et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat. Commun. 8, 1–11 (2017).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D. 67, 293–302 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. 66, 486–501 (2010).
Smart, O. S. et al. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in BUSTER. Acta Crystallogr. D. 68, 368–380 (2012).
Meerbrey, K. L. et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc. Natl Acad. Sci. USA 108, 3665–3670 (2011).
Lionnet, T. et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat. Methods 8, 165–170 (2011).
Rosenbloom, K. R. et al. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 43, D670–D681 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Acknowledgements
We thank H. Großhans, T.S. Miki, D. Porter, R. Tiedt, J. Baryza and G. Rice for helpful discussions through the course of this research study. This work was supported by the Novartis Research Foundation (J.A.C.), the SNF-NCCR RNA & Disease network (J.A.C.) and the Medical Research Council (MRC) grant no. MC_U105192715 (L.A.P.).
Author information
Authors and Affiliations
Contributions
R.E.J.B., F.L. and N.T.R conceptualized research, interpreted data and wrote the manuscript. S.C. conducted and interpreted biomarker, variomics and MoA studies. A.F. synthesized all compounds. W.A.W. conducted crystallography experiments and solved structures. M.H. conducted the siRNA synergy experiment. M.S. and G.M. conducted and interpreted CPSF3 biochemical binding studies. J.T., S.C. and F.A.A. prepared RNA samples. J.K. and W.C. conducted RNA-sequencing experiments. S.H.C., S.G., S.S. and G.R. interpreted RNA-seq results. S.B., J.M., J.T. and M.S. conducted and interpreted chemical proteomics experiments. G.M. and S.C. conducted and interpreted FACS experiments. Y.W. and J.J. contributed to in silico target predictions. F.S. analyzed siRNA experiments. H.M. conducted cell line cytotoxicity studies. G.B. and E.G. conducted and interpreted in vivo xenograft experiments. A.C. and K.X. conducted variomics cloning experiments. J.S.R.-H. designed variomics cloning strategy. M.Z. and M.S. conducted mass spectrometry experiments. C.S. and E.T.W. conducted in vivo compound pharmacokinetics experiments. J.H.W., C.A.-R., M.J. and J.A.C. conducted and interpreted FISH and R-loop experiments. J.B.R.M. and L.A.P. conducted and interpreted in vitro cleavage experiments. J.A.C. and J.T. helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors (except otherwise noted) were employees of Novartis Institutes for BioMedical Research at the time of their involvement in this study and may hold stock in Novartis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figures 1–21 and Supplementary Tables 1–4
Supplementary Note
Synthetic Procedures
Supplementary Dataset 1
siRNA library.
Supplementary Dataset 2
siRNA plus compound 1 synergy screen gene level activity per compound 1, DMSO or differential.
Supplementary Dataset 3
Chemoproteomics data.
Supplementary Dataset 4
PAL data.
Rights and permissions
About this article
Cite this article
Ross, N.T., Lohmann, F., Carbonneau, S. et al. CPSF3-dependent pre-mRNA processing as a druggable node in AML and Ewing’s sarcoma. Nat Chem Biol 16, 50–59 (2020). https://doi.org/10.1038/s41589-019-0424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0424-1
This article is cited by
-
Splicing targeting drugs highlight intron retention as an actionable vulnerability in advanced prostate cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
RBBP6 maintains glioblastoma stem cells through CPSF3-dependent alternative polyadenylation
Cell Discovery (2024)
-
Cleavage and polyadenylation machinery as a novel targetable vulnerability for human cancer
Cancer Gene Therapy (2024)
-
A promising natural product in diffuse large B-cell lymphoma therapy by targeting PIM1
Annals of Hematology (2024)
-
The anticancer compound JTE-607 reveals hidden sequence specificity of the mRNA 3′ processing machinery
Nature Structural & Molecular Biology (2023)