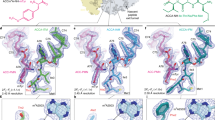
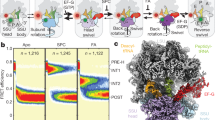
Abstract
Chloramphenicol (CHL) and linezolid (LZD) are antibiotics that inhibit translation. Both were thought to block peptide-bond formation between all combinations of amino acids. Yet recently, a strong nascent peptide context-dependency of CHL- and LZD-induced translation arrest was discovered. Here we probed the mechanism of action of CHL and LZD by using single-molecule Förster resonance energy transfer spectroscopy to monitor translation arrest induced by antibiotics. The presence of CHL or LZD does not substantially alter dynamics of protein synthesis until the arrest-motif of the nascent peptide is generated. Inhibition of peptide-bond formation compels the fully accommodated A-site transfer RNA to undergo repeated rounds of dissociation and nonproductive rebinding. The glycyl amino-acid moiety on the A-site Gly-tRNA manages to overcome the arrest by CHL. Our results illuminate the mechanism of CHL and LZD action through their interactions with the ribosome, the nascent peptide and the incoming amino acid, perturbing elongation dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All experimental data are available upon reasonable request.
Code availability
All MATLAB scripts used in this study are available upon request.
References
Rodnina, M. V. & Wintermeyer, W. Peptide bond formation on the ribosome: structure and mechanism. Curr. Opin. Struct. Biol. 13, 334–340 (2003).
Frank, J. & Agrawal, R. K. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406, 318–322 (2000).
Valle, M. et al. Locking and unlocking of ribosomal motions. Cell 114, 123–134 (2003).
Agirrezabala, X. et al. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol. Cell 32, 190–197 (2008).
Dunkle, J. A. et al. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332, 981–984 (2011).
Ermolenko, D. N. et al. Observation of intersubunit movement of the ribosome in solution using FRET. J. Mol. Biol. 370, 530–540 (2007).
Moazed, D. & Noller, H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature 342, 142–148 (1989).
Munro, J. B., Altman, R. B., O’Connor, N. & Blanchard, S. C. Identification of two distinct hybrid state intermediates on the ribosome. Mol. Cell 25, 505–517 (2007).
Bock, L. V. et al. Energy barriers and driving forces in tRNA translocation through the ribosome. Nat. Struct. Mol. Biol. 20, 1390–1396 (2013).
Jamiolkowski, R. M., Chen, C., Cooperman, B. S. & Goldman, Y. E. tRNA fluctuations observed on stalled ribosomes are suppressed during ongoing protein synthesis. Biophys. J. 113, 2326–2335 (2017).
Voorhees, R. M. & Ramakrishnan, V. Structural basis of the translational elongation cycle. Annu. Rev. Biochem. 82, 203–236 (2013).
Polacek, N. & Mankin, A. S. The ribosomal peptidyl transferase center: structure, function, evolution, inhibition. Crit. Rev. Biochem. Mol. Biol. 40, 285–311 (2005).
Wilson, D. N. The A-Z of bacterial translation inhibitors. Crit. Rev. Biochem. Mol. Biol. 44, 393–433 (2009).
Lin, J., Zhou, D., Steitz, T. A., Polikanov, Y. S. & Gagnon, M. G. Ribosome-targeting antibiotics: modes of action, mechanisms of resistance, and implications for drug design. Annu. Rev. Biochem. 87, 451–478 (2018).
Arenz, S. & Wilson, D. N. Bacterial protein synthesis as a target for antibiotic inhibition. Cold Spring Harb. Perspect. Med. 6, a025361 (2016).
Ehrlich, J., Gottlieb, D., Burkholder, P. R., Andierson, L. E. & Pridham, T. G. Streptomyces venezuelae, n. sp., the source of chloromycetin. J. Bacteriol. 56, 467–477 (1948).
Brickner, S. J., Barbachyn, M. R., Hutchinson, D. K. & Manninen, P. R. Linezolid (ZYVOX), the first member of a completely new class of antibacterial agents for treatment of serious Gram-positive infections. J. Med. Chem. 51, 1981–1990 (2008).
Schlunzen, F. et al. Structural basis for the interaction of antibiotics with the peptidyl transferase center in eubacteria. Nature 413, 814–821 (2001).
Leach, K. L. et al. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 26, 393–402 (2007).
Wilson, D. N. et al. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl Acad. Sci. USA 105, 13339–13344 (2008).
Bulkley, D., Innis, C. A., Blaha, G. & Steitz, T. A. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc. Natl Acad. Sci. USA 107, 17158–17163 (2010).
Pestka, S. in Antibitoics III: Mechanism of Action of Antimicrobial and Antitumor Agents (eds Corcoran, J. W. & Hahn, F. E.) 370–395 (Springer-Verlag, 1975).
Marks, J. et al. Context-specific inhibition of translation by ribosomal antibiotics targeting the peptidyl transferase center. Proc. Natl Acad. Sci. USA 113, 12150–12155 (2016).
Polikanov, Y. S. et al. Distinct tRNA accommodation intermediates observed on the ribosome with the antibiotics hygromycin A and A201A. Mol. Cell 58, 832–844 (2015).
Orelle, C. et al. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob. Agents Chemother. 57, 5994–6004 (2013).
Aitken, C. E. & Puglisi, J. D. Following the intersubunit conformation of the ribosome during translation in real time. Nat. Struct. Mol. Biol. 17, 793–800 (2010).
Dorywalska, M. et al. Site-specific labeling of the ribosome for single-molecule spectroscopy. Nucleic Acids Res. 33, 182–189 (2005).
Uemura, S. et al. Real-time tRNA transit on single translating ribosomes at codon resolution. Nature 464, 1012–1017 (2010).
Chen, J., Tsai, A., Petrov, A. & Puglisi, J. D. Nonfluorescent quenchers to correlate single-molecule conformational and compositional dynamics. J. Am. Chem. Soc. 134, 5734–5737 (2012).
Choi, J. & Puglisi, J. D. Three tRNAs on the ribosome slow translation elongation. Proc. Natl Acad. Sci. USA 114, 13691–13696 (2017).
Blanchard, S. C., R. L., Kim, H. D., Chu, S. & Puglisi, J. D. tRNA selection and kinetic proofreading in translation. Nat. Struct. Mol. Biol. 11, 1008–1014 (2004).
Choi, J. et al. N6-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat. Struct. Mol. Biol. 23, 110–115 (2016).
Choi, J. et al. 2′-O-methylation in mRNA disrupts tRNA decoding during translation elongation. Nat. Struct. Mol. Biol. 25, 208–216 (2018).
Chen, J. et al. High-throughput platform for real-time monitoring of biological processes by multicolor single-molecule fluorescence. Proc. Natl Acad. Sci. USA 111, 664–669 (2014).
Wasserman, M. R., Alejo, J. L., Altman, R. B. & Blanchard, S. C. Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat. Struct. Mol. Biol. 23, 333–341 (2016).
Loveland, A. B., Demo, G., Grigorieff, N. & Korostelev, A. A. Ensemble cryo-EM elucidates the mechanism of translation fidelity. Nature 546, 113–117 (2017).
Alejo, J. L. & Blanchard, S. C. Miscoding-induced stalling of substrate translocation on the bacterial ribosome. Proc. Natl Acad. Sci. USA. 114, E8603–E8610 (2017).
Marshall, R. A., Aitken, C. E. & Puglisi, J. D. GTP hydrolysis by IF2 guides progression of the ribosome into elongation. Mol. Cell 35, 37–47 (2009).
Fislage, M. et al. Cryo-EM shows stages of initial codon selection on the ribosome by aa-tRNA in ternary complex with GTP and the GTPase-deficient EF-TuH84A. Nucleic Acids Res. 46, 5861–5874 (2018).
Brilot, A. F., Korostelev, A. A., Ermolenko, D. N. & Grigorieff, N. Structure of the ribosome with elongation factor G trapped in the pretranslocation state. Proc. Natl Acad. Sci. USA 110, 20994–20999 (2013).
Murakami, H., Ohta, A., Ashigai, H. & Suga, H. A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat. Methods 3, 357–359 (2006).
Goto, Y. & Suga, H. Translation initiation with initiator tRNA charged with exotic peptides. J. Am. Chem. Soc. 131, 5040–5041 (2009).
Dunkle, J. A., Xiong, L., Mankin, A. S. & Cate, J. H. D. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl Acad. Sci. USA 107, 17152–17157 (2010).
Ippolito, J. A. et al. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 51, 3353–3356 (2008).
Polikanov, Y. S., Steitz, T. A. & Innis, C. A. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat. Struct. Mol. Biol. 21, 787–793 (2014).
Huter, P. et al. Structural basis for polyproline-mediated ribosome stalling and rescue by the translation elongation factor EF-P. Mol. Cell 68, 515–527.e6 (2017).
Thompson, J., O’Connor, M., Mills, J. A. & Dahlberg, A. E. The protein synthesis inhibitors, oxazolidinones and chloramphenicol, cause extensive translational inaccuracy in vivo. J. Mol. Biol. 322, 273–279 (2002).
Vazquez-Laslop, N., Thum, C. & Mankin, A. S. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell 30, 190–202 (2008).
Orelle, C. et al. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 41, e144 (2013).
Wang, J., Kwiatkowski, M. & Forster, A. C. Kinetics of ribosome-catalyzed polymerization using artificial aminoacyl-tRNA substrates clarifies inefficiencies and improvements. ACS Chem. Biol. 10, 2187–2192 (2015).
Acknowledgements
This work was supported by the US National Institutes of Health (grant nos. GM51266 to J.D.P. and R01 AI125518 to A.S.M. and N. V.-L.); by a Stanford Bio-X fellowship to J.C.; by the Knut and Alice Wallenberg Foundation postdoctoral scholarships to J.Z. and to J.W. (no. 2015.0406). We are grateful to H. Suga for providing the substrate for the flexizyme reaction and C. Innis for the advice and training in using the flexizyme-based procedures. We thank members of the Puglisi laboratory and the Mankin/Vázquez-Laslop laboratory for discussion and input.
Author information
Authors and Affiliations
Contributions
J.C., J.M., N.V.-L., A.S.M. and J.D.P. conceived the project and designed the single-molecule experiments. J.M. performed the toeprinting assay. J.C. prepared the reagent, and performed the single-molecule experiment and data analysis with the help of J.M. and J.W. J.Z. and D.-H.C. provided supporting data. J.C., J.M., J.Z., N.V.-L., A.S.M. and J.D.P. wrote the manuscript with the input of others.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figs. and Tables
Supplementary Figs. 1–4.
Source Data for Main Figures
Source Data for Main Figures
Rights and permissions
About this article
Cite this article
Choi, J., Marks, J., Zhang, J. et al. Dynamics of the context-specific translation arrest by chloramphenicol and linezolid. Nat Chem Biol 16, 310–317 (2020). https://doi.org/10.1038/s41589-019-0423-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0423-2
This article is cited by
-
Euglena mutabilis exists in a FAB consortium with microbes that enhance cadmium tolerance
International Microbiology (2024)
-
Atlas of mRNA translation and decay for bacteria
Nature Microbiology (2023)
-
Putting the antibiotics chloramphenicol and linezolid into context
Nature Structural & Molecular Biology (2022)
-
Structural basis for the context-specific action of the classic peptidyl transferase inhibitor chloramphenicol
Nature Structural & Molecular Biology (2022)
-
Structural basis for PoxtA-mediated resistance to phenicol and oxazolidinone antibiotics
Nature Communications (2022)