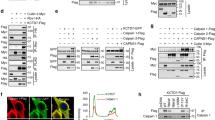
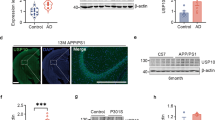
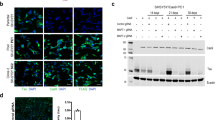
Abstract
Protein–protein interactions between E3 ubiquitin ligases and protein termini help shape the proteome. These interactions are sensitive to proteolysis, which alters the ensemble of cellular N and C termini. Here we describe a mechanism wherein caspase activity reveals latent C termini that are then recognized by the E3 ubiquitin ligase CHIP. Using expanded knowledge of CHIP’s binding specificity, we predicted hundreds of putative interactions arising from caspase activity. Subsequent validation experiments confirmed that CHIP binds the latent C termini at tauD421 and caspase-6D179. CHIP binding to tauD421, but not tauFL, promoted its ubiquitination, while binding to caspase-6D179 mediated ubiquitin-independent inhibition. Given that caspase activity generates tauD421 in Alzheimer’s disease (AD), these results suggested a concise model for CHIP regulation of tau homeostasis. Indeed, we find that loss of CHIP expression in AD coincides with the accumulation of tauD421 and caspase-6D179. These results illustrate an unanticipated link between caspases and protein homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All structural data has been deposited in the PDB (PDB ID 6EFK and 6NSV). All predicted CHIP binders and relevant scores are provided in the Supplementary Information. Additional data supporting the findings of this manuscript are available from the corresponding author upon reasonable request.
References
Dougan, D. A., Micevski, D. & Truscott, K. N. The N-end rule pathway: From recognition by N-recognins, to destruction by AAA+ proteases. Biochim. Biophys. Acta—Mol. Cell Res. 1823, 83–91 (2012).
Tonikian, R. et al. A specificity map for the PDZ domain family. PLoS Biol. 6, 2043–2059 (2008).
Dong, C. et al. Molecular basis of GID4-mediated recognition of degrons for the Pro/N-end rule pathway article. Nat. Chem. Biol. 14, 466–473 (2018).
Zhang, C. et al. High-resolution crystal structure of human protease-activated receptor 1. Nature 492, 387–392 (2012).
Saelens, X. et al. Toxic proteins released from mitochondria in cell death. Oncogene 23, 2861–2874 (2004).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Zheng, N. & Shabek, N. Ubiquitin ligases: structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017).
Bachmair, A., Finley, D. & Varshavsky, A. In vivo half-life of a protein is a function of its amino-terminal residue. Science 234, 179–186 (1986).
Chen, S.-J., Wu, X., Wadas, B., Oh, J.-H. & Varshavsky, A. An N-end rule pathway that recognizes proline and destroys gluconeogenic enzymes. Science 355, eaal3655 (2017).
Kim, H. K. et al. The N-terminal methionine of cellular proteins as a degradation signal. Cell 156, 158–169 (2014).
Varshavsky, A. The N-end rule pathway and regulation by proteolysis. Protein Sci. 20, 1298–1345 (2011).
Koren, I. et al. The eukaryotic proteome is shaped by E3 ubiquitin ligases targeting C-terminal degrons. Cell 173, 1622–1635 (2018).
Lin, H. et al. C-terminal end-directed protein elimination by CRL2 ubiquitin ligases. Mol. Cell 70, 602–613.e3 (2018).
Scott, F. L. et al. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionary conserved mechanism of IAPs. EMBO J. 24, 645–655 (2005).
Saita, S. et al. PARL mediates Smac proteolytic maturation in mitochondria to promote apoptosis. Nat. Cell Biol. 19, 318–328 (2017).
Wu, G. et al. Structural basis of IAP recognition by Smac/DIABLO. Nature 408, 1008–1012 (2000).
Ballinger, C. A. et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19, 4535–4545 (1999).
Jiang, J. et al. CHIP is a U-box-dependent E3 ubiquitin ligase. J. Biol. Chem. 276, 42938–42944 (2001).
Zhang, M. et al. Chaperoned ubiquitylation—crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol. Cell 20, 653–659 (2005).
Qian, S. B., McDonough, H., Boellmann, F., Cyr, D. M. & Patterson, C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature 440, 551–555 (2006).
Rodina, A. et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 538, 397–401 (2016).
Taipale, M. et al. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 158, 434–448 (2014).
Wang, L. et al. Molecular mechanism of the negative regulation of Smad1/5 protein by carboxyl terminus of Hsc70-interacting protein (CHIP). J. Biol. Chem. 286, 15883–15894 (2011).
Assimon, V. A., Southworth, D. R. & Gestwicki, J. E. Specific binding of tetratricopeptide repeat proteins to heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90) is regulated by affinity and phosphorylation. Biochemistry 54, 7120–7131 (2015).
Choe, Y. et al. Substrate profiling of cysteine proteases using a combinatorial peptide library identifies functionally unique specificities. J. Biol. Chem. 281, 12824–12832 (2006).
Brinker, A. et al. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70·Hop·Hsp90 complexes. J. Biol. Chem. 277, 19265–19275 (2002).
Scheufler, C. et al. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101, 199–210 (2000).
Kellogg, E. H., Leaver-Fay, A. & Baker, D. Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins Struct. Funct. Bioinforma 79, 830–838 (2011).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-l fJ processing in monocytes. Nature 356, 768–774 (1992).
Julien, O. & Wells, J. A. Caspases and their substrates. Cell Death Differ. 24, 1380–1389 (2017).
Crawford, E. D. et al. The DegraBase: a database of proteolysis in healthy and apoptotic human cells. Mol. Cell. Proteomics 12, 813–824 (2012).
Barkan, D. T. et al. Prediction of protease substrates using sequence and structure features. Bioinformatics 26, 1714–1722 (2010).
Stennicke, H. R. & Salvesen, G. S. Caspases—controlling intracellular signals by protease zymogen activation. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 1477, 299–306 (2000).
Rissman, R. A. et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J. Clin. Invest. 114, 121–130 (2004).
Guo, H. et al. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am. J. Pathol. 165, 523–531 (2004).
Theofilas, P. et al. Probing the correlation of neuronal loss, neuro fibrillary tangles, and cell death markers across the Alzheimer’s disease Braak stages: a quantitative study in humans. Neurobiol. Aging 61, 1–12 (2018).
Dolan, P. J. & Johnson, G. V. W. A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J. Biol. Chem. 285, 21978–21987 (2010).
Saidi, L.-J. et al. Carboxy terminus heat shock protein 70 interacting protein reduces tau-associated degenerative changes. J. Alzheimer’s Dis. 44, 937–947 (2015).
Dickey, C. A. et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J. Neurosci. 26, 6985–6996 (2006).
Kellogg, E. H. et al. Near-atomic model of microtubule-tau interactions. Science 360, 1242–1246 (2018).
Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017).
Morris, M. et al. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 18, 1183–1189 (2015).
Quinn, J. P., Corbett, N. J., Kellett, K. A. B. & Hooper, N. M. Tau proteolysis in the pathogenesis of tauopathies: neurotoxic fragments and novel biomarkers. J. Alzheimer’s Dis. 63, 1–21 (2018).
Deveraux, Q. L., Takahashi, R., Salvesen, G. S. & Reed, J. C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388, 300–304 (1997).
Braak, H., Braak, E. & Bohl, J. Staging of Alzheimer-related cortical destruction. Eur. Neurol. 33, 403–408 (1993).
J.A., M. et al. Neuropathological and transcriptomic characteristics of the aged brain. eLife 6, 1–26 (2017).
Riedl, S. J. & Salvesen, G. S. The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 8, 405–413 (2007).
Klaiman, G., Champagne, N. & Leblanc, A. C. Self-activation of Caspase-6 in vitro and in vivo: Caspase-6 activation does not induce cell death in HEK293T cells. Biochem Biophys. Acta 1793, 592–601 (2009).
Dagbay, K. B. & Hardy, J. A. Multiple proteolytic events in caspase-6 self-activation impact conformations of discrete structural regions. Proc. Natl Acad. Sci. USA 114, E7977–E7986 (2017).
Julien, O. et al. Quantitative MS-based enzymology of caspases reveals distinct protein substrate specificities, hierarchies, and cellular roles. Proc. Natl Acad. Sci. USA 113, E2001–E2010 (2016).
Nikolovska-Coleska, Z. et al. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 332, 261–273 (2004).
Winter, G. Xia2: An expert system for macromolecular crystallography data reduction. J. Appl. Crystallogr. 43, 186–190 (2010).
Long, F., Vagin, A. A., Young, P. & Murshudov, G. N. BALBES: a molecular-replacement pipeline. Acta Crystallogr. Sect. D. 64, 125–132 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D. 66, 213–221 (2010).
Grinberg, L. T. et al. Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 8, 151–162 (2007).
Acknowledgements
This work is dedicated to R. Raines on the occasion of his 60th birthday. This work was supported by grants from the Tau Consortium and NIH no. R01059690 (to J.E.G.), nos. P41CA196276 and P50GM082250 (to C.S.C.), no. K24 AG053435 (to L.T.G.) Alzheimer Association grant no. AARG-16-441514 (to L.T.G. and M.A.). Additional support included an NSF GRFP fellowship (to K.A.O.-N.), an ARCS Foundation fellowship (to M.R.), institutional grant nos. UL1 TR001872 and K01AG053433 (to P.T.) and a Program for Breakthrough Biomedical Science funded by the Sandler Foundation (to C.S.C.). The authors thank the laboratories of K.M. Scaglione (Medical College of Wisconsin) and J. A. Wells (University of California San Francisco) for technical support, as well as S.-A. Mok for input on the manuscript.
Author information
Authors and Affiliations
Contributions
M.R., C.S.C. and J.E.G. designed the studies and wrote the manuscript. M.R., K.A.O.-N., V.A.A., Y.-F.C. and D.M.-C. conducted biochemistry experiments and generated necessary reagents. K.B., M.F.B. and M.R. designed and executed structural and computational studies. M.R. and D.M.-C. designed and conducted cell biology experiments. P.T. and L.T.G designed and conducted the immunohistochemistry experiments. J.E.G., C.S.C., M.A. and L.T.G. provided funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Tables 1–5, Supplementary Figures 1–9
Supplementary Tables 1–5, Supplementary Figs. 1–9
Supplementary Table S5
List of caspase substrates that include potential, cryptic CHIP-binding sequences.
Rights and permissions
About this article
Cite this article
Ravalin, M., Theofilas, P., Basu, K. et al. Specificity for latent C termini links the E3 ubiquitin ligase CHIP to caspases. Nat Chem Biol 15, 786–794 (2019). https://doi.org/10.1038/s41589-019-0322-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0322-6
This article is cited by
-
Exploring the significance of caspase-cleaved tau in tauopathies and as a complementary pathology to phospho-tau in Alzheimer’s disease: implications for biomarker development and therapeutic targeting
Acta Neuropathologica Communications (2024)
-
Mirror-image ligand discovery enabled by single-shot fast-flow synthesis of D-proteins
Nature Communications (2024)
-
Rare antibody phage isolation and discrimination (RAPID) biopanning enables identification of high-affinity antibodies against challenging targets
Communications Biology (2023)
-
Recognition and reprogramming of E3 ubiquitin ligase surfaces by α-helical peptides
Nature Communications (2023)
-
STUB1 is an intracellular checkpoint for interferon gamma sensing
Scientific Reports (2022)