Abstract
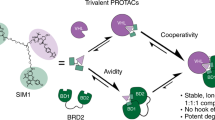
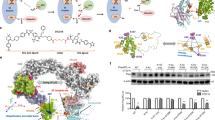
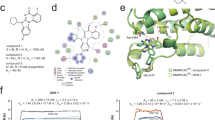
Targeting subunits of BAF/PBAF chromatin remodeling complexes has been proposed as an approach to exploit cancer vulnerabilities. Here, we develop proteolysis targeting chimera (PROTAC) degraders of the BAF ATPase subunits SMARCA2 and SMARCA4 using a bromodomain ligand and recruitment of the E3 ubiquitin ligase VHL. High-resolution ternary complex crystal structures and biophysical investigation guided rational and efficient optimization toward ACBI1, a potent and cooperative degrader of SMARCA2, SMARCA4 and PBRM1. ACBI1 induced anti-proliferative effects and cell death caused by SMARCA2 depletion in SMARCA4 mutant cancer cells, and in acute myeloid leukemia cells dependent on SMARCA4 ATPase activity. These findings exemplify a successful biophysics- and structure-based PROTAC design approach to degrade high profile drug targets, and pave the way toward new therapeutics for the treatment of tumors sensitive to the loss of BAF complex ATPases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Atomic coordinates and structure factors for new protein structures SMARCA2BD:SMARCABD, SMARCA2BD:PROTAC 1:VCB, SMARCA2BD:PROTAC 2:VCB and SMARCA4BD:PROTAC 2:VCB have been deposited in the PDB under accession numbers 6HAZ, 6HAY, 6HAX and 6HR2, respectively. All data generated and analyzed during this study are included in this published article and its Supplementary Information or are available from the corresponding authors upon reasonable request.
Change history
02 July 2019
In the version of this article originally published, several lines of text in the last paragraph of the right column on page 1 of the PDF were transposed into the bottom paragraph of the left column. The affected text of the left column should read “The ATP-dependent activities of the BAF (SWI/SNF) chromatin remodeling complexes affect the positioning of nucleosomes on DNA and thereby many cellular processes related to chromatin structure, including transcription, DNA repair and decatenation of chromosomes during mitosis12,13.” The affected text of the right column should read “SMARCA2/4BD inhibitors are thus precluded from use for the treatment of SMARCA4 mutant cancers but could provide attractive ligands for PROTAC conjugation. Small molecules binding to other bromodomains have been successfully converted into PROTACs by conjugating them with structures capable of binding to the E3 ligases von Hippel−Lindau (VHL) or cereblon5,6,10,11,25,26,27.” The errors have been corrected in the PDF version of the paper.
References
Toure, M. & Crews, C. M. Small-molecule PROTACS: new approaches to protein degradation. Angew. Chemie Int. 55, 1966–1973 (2016).
Collins, I., Wang, H., Caldwell, J. J. & Chopra, R. Chemical approaches to targeted protein degradation through modulation of the ubiquitin–proteasome pathway. Biochem. J. 474, 1127–1147 (2017).
Hughes, S. J. & Ciulli, A. Molecular recognition of ternary complexes: a new dimension in the structure-guided design of chemical degraders. Essays Biochem. 61, 505–516 (2017).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015).
Winter, G. E. et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015).
Zengerle, M., Chan, K.-H. & Ciulli, A. Selective small molecule induced degradation of the BET bromodomain protein BRD4. ACS Chem. Biol. 10, 1770–1777 (2015).
Gadd, M. S. et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat.Chem. Biol. 13, 514–521 (2017).
Nowak, R. P. et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol. 14, 706–714 (2018).
Bondeson, D. P. et al. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem. Biol. 25, 78–87. e75 (2018).
Gechijian, L. N. et al. Functional TRIM24 degrader via conjugation of ineffectual bromodomain and VHL ligands. Nat. Chem. Biol. 14, 405–412 (2018).
Bassi, Z. I. et al. Modulating PCAF/GCN5 immune cell function through a PROTAC approach. ACS Chem. Biol. 13, 2862–2867 (2018).
Kadoch, C. & Crabtree, G. R. Mammalian SWI/SNF chromatin remodeling complexes and cancer: mechanistic insights gained from human genomics. Sci. Adv. 1, e1500447 (2015).
St Pierre, R. & Kadoch, C. Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Curr. Opin. Genet. Dev. 42, 56–67 (2017).
Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013).
Hodges, C., Kirkland, J. G. & Crabtree, G. R. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med. 6, a026930 (2016).
Shain, A. H. & Pollack, J. R. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One 8, e55119 (2013).
Shi, J. et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev. 27, 2648–2662 (2013).
Hoffman, G. R. et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc. Natl Acad. Sci. USA 111, 3128–3133 (2014).
Oike, T. et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res. 73, 5508–5518 (2013).
Wilson, B. G. et al. Residual complexes containing SMARCA2 (BRM) underlie the oncogenic drive of SMARCA4 (BRG1) mutation. Mol. Cell. Biol. 34, 1136–1144 (2014).
Papillon, J. P. N. et al. Discovery of orally active inhibitors of brahma homolog (BRM)/SMARCA2 ATPase activity for the treatment of brahma related gene 1 (BRG1)/SMARCA4-mutant cancers. J. Med. Chem. 61, 10155–10172 (2018).
Sutherell, C. L. et al. Identification and development of 2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-one inhibitors targeting bromodomains within the switch/sucrose nonfermenting complex. J. Med. Chem. 59, 5095–5101 (2016).
Gerstenberger, B. S. et al. Identification of a chemical probe for family VIII bromodomains through optimization of a fragment hit. J. Med. Chem. 59, 4800–4811 (2016).
Vangamudi, B. et al. The SMARCA2/4 ATPase domain surpasses the bromodomain as a drug target in SWI/SNF-mutant cancers: insights from cDNA rescue and PFI-3 inhibitor studies. Cancer Res. 75, 3865–3878 (2015).
Remillard, D. et al. Degradation of the BAF complex factor BRD9 by heterobifunctional ligands. Angew. Chemie Int. 56, 5738–5743 (2017).
Zoppi, V. et al. Iterative design and optimization of initially inactive proteolysis targeting chimeras (PROTACs) identify VZ185 as a potent, fast, and selective von hippel–lindau (VHL) based dual degrader probe of BRD9 and BRD7. J. Med. Chem. 62, 699–726 (2019).
Qin, C. et al. Discovery of QCA570 as an exceptionally potent and efficacious proteolysis targeting chimera (PROTAC) degrader of the bromodomain and extra-terminal (BET) proteins capable of inducing complete and durable tumor regression. J. Med. Chem. 61, 6685–6704 (2018).
Albrecht, B. K. et al. Therapeutic pyridazine compounds and uses thereof. WIPO patent WO2016138114 (2016).
Myrianthopoulos, V. et al. Discovery and optimization of a selective ligand for the switch/sucrose nonfermenting-related bromodomains of polybromo protein-1 by the use of virtual screening and hydration analysis. J. Med. Chem. 59, 8787–8803 (2016).
Soares, P. et al. Group-based optimization of potent and cell-active inhibitors of the von Hippel-Lindau (VHL) E3 ubiquitin ligase: structure-activity relationships leading to the chemical probe (2S,4R)-1-((S)-2-(1-cyanocyclopropanecarboxamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (VH298). J. Med. Chem. 61, 599–618 (2018).
Buckley, D. L. et al. HaloPROTACS: use of small molecule PROTACS to induce degradation of halotag fusion proteins. ACS Chem. Biol. 10, 1831–1837 (2015).
Maniaci, C. et al. Homo-PROTACs: bivalent small-molecule dimerizers of the VHL E3 ubiquitin ligase to induce self-degradation. Nat. Commun. 8, 830 (2017).
Chan, K.-H., Zengerle, M., Testa, A. & Ciulli, A. Impact of target warhead and linkage vector on inducing protein degradation: comparison of bromodomain and extra-terminal (BET) degraders derived from triazolodiazepine (JQ1) and tetrahydroquinoline (I-BET726) BET inhibitor scaffolds. J. Med. Chem. 61, 504–513 (2018).
Van Molle, I. et al. Dissecting fragment-based lead discovery at the von Hippel–Lindau protein:Hypoxia inducible factor 1α protein–protein interface. Chem. Biol. 19, 1300–1312 (2012).
Soucy, T. A. et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732 (2009).
Zorba, A. et al. Delineating the role of cooperativity in the design of potent PROTACs for BTK. Proc. Natl Acad. Sci. USA 115, E7285–E7292 (2018).
Zhao, K. et al. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 95, 625–636 (1998).
Qiu, Z. & Ghosh, A. A calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron 60, 775–787 (2008).
McDonald, E. R. et al. Project DRIVE: a compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell 170, 577–592.e510 (2017).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e516 (2017).
Reyes, J. C. et al. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2α). EMBO J. 17, 6979–6991 (1998).
Bultman, S. et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell 6, 1287–1295 (2000).
Fedorov, O. et al. Selective targeting of the BRG/PB1 bromodomains impairs embryonic and trophoblast stem cell maintenance. Science Adv. 1, e1500723 (2015).
Filippakopoulos, P. et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231 (2012).
Vonrhein, C. et al. Data processing and analysis with the auto PROC toolbox. Acta Crystallog. D Biol. Crystallogr. 67, 293–302 (2011).
Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr 66, 12–21 (2009).
Bruno, I. J. et al. Retrieval of crystallographically-derived molecular geometry information. J. Chem. Inf. Comput. Sci. 44, 2133–2144 (2004).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013).
Hughes, C. S. et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 10, 757–757 (2014).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. R. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Acknowledgements
This project has received funding from Boehringer Ingelheim. A.C. was partially supported by a European Research Council (ERC) Starting Grant (grant agreement no. ERC-2012-StG-311460 DrugE3CRLs). Biophysics, drug discovery and proteomics/computing activities at Dundee were partially supported by Wellcome Trust strategic awards (nos. 100476/Z/12/Z, 094090/Z/10/Z and 097945/C/11/Z, respectively). We are thankful to D. Covini for synthesis support; G. Glendinning and S. Mayer for compound logistics; D. Haering and A. Weiss for help with cooperativity and protein degradation measurements; S. Winkler and M. Scharnweber for help with SPR measurements; K. Mayr for LogD measurements; S. Doebel, G. Flotzinger, G. Siszler and P. Werni for protein production and purification; P. Fyfe for support with the in-house X-ray facility; M. Gadd for the gift of the VHLR69A construct; D. Lamont for assistance in proteomics and V. Vetma for data analysis. We also thank the Diamond Light Source for beamtime (BAG proposal MX14980) and for beamline support at beamline IO4-1 and I24.
Author information
Authors and Affiliations
Contributions
A.C., M.P. and M.K. conceived the idea. A.C., W.F., M.K., S.S., M.P., D.B.M. and P.E. directed the project. W.F., S.S. and C.D. designed compounds. M.K., A.C., W.F., T.O.H., C.W., N.T., N.W., E.D., H.W., C.D., B.S., S.W. and M.J.R. designed experiments and interpreted data. E.D., N.T., J.K.O., O.P. and P.G. synthesized compounds. Crystallography was performed by M.J.R., G. Bader and A.W. P. G. Boehmelt helped in the design of a cell line used for testing PROTAC-induced protein degradation. Protein production was performed and supervised by M.J.R. and A.Z. T. Gerstberger supervised TR–FRET competition experiments, B.S. performed TR–FRET competition experiments. K.R. supervised SPR experiments, M.G. performed SPR experiments. E.T. and C.W. performed degradation studies supervised by R.S. and M.K. T. Gmaschitz, J.W., K.E.-W. and C.R. performed overexpression studies, proliferation, degradation and apoptosis assays. M.J.R. and D.Z. developed and performed fluorescence polarization cooperativity assays and M.J.R. performed the ITC cooperativity assays. C.W. developed and performed the cell lysate AlphaLISA assay. N.T. and N.W. performed MS proteomics. M.Y.W. developed IP–MS protocols. J.R. and H.A. supervised in vitro drug metabolism and pharmacokinetics experiments. W.F. and M.K. wrote the manuscript with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
A.C. is a scientific founder, director and shareholder of Amphista Therapeutics, a company that is developing targeted protein degradation therapeutic platforms.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, Supplementary Figures 1–15
Supplementary Note
Synthetic Procedures
Source data
Dataset 1
Proteomic analysis of relative protein abundance in MV-4-11 cells using ACBI1.
Dataset 2
Proteomic analysis of relative protein abundance in MV-4-11 cells using PROTAC 2.
Dataset 3
IP–MS data representing protein abundance in MV-4-11 cells using PROTAC 2.
Dataset 4
IP–MS data representing protein abundance in MV-4-11 cells using PROTAC 2, including IgG controls.
Rights and permissions
About this article
Cite this article
Farnaby, W., Koegl, M., Roy, M.J. et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat Chem Biol 15, 672–680 (2019). https://doi.org/10.1038/s41589-019-0294-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0294-6
This article is cited by
-
Pooled multicolour tagging for visualizing subcellular protein dynamics
Nature Cell Biology (2024)
-
Ubiquitin-specific proximity labeling for the identification of E3 ligase substrates
Nature Chemical Biology (2024)
-
Context-specific functions of chromatin remodellers in development and disease
Nature Reviews Genetics (2024)
-
DNA-encoded library-enabled discovery of proximity-inducing small molecules
Nature Chemical Biology (2024)
-
Development and crystal structures of a potent second-generation dual degrader of BCL-2 and BCL-xL
Nature Communications (2024)