Abstract
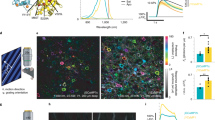
Fluorescent indicators are used widely to visualize calcium dynamics downstream of membrane depolarization or G-protein-coupled receptor activation, but are poorly suited for non-invasive imaging in mammals. Here, we report a bright calcium-modulated bioluminescent indicator named Orange CaMBI (Orange Calcium-modulated Bioluminescent Indicator). Orange CaMBI reports calcium dynamics in single cells and, in the context of a transgenic mouse, reveals calcium oscillations in whole organs in an entirely non-invasive manner.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Nucleotide sequences are available at GenBank for Orange CaMBI 110 (MK558047), Orange CaMBI 300 (MK558048), Blue CaMBI (MK558049), and Green CaMBI (MK558050). Mammalian expression plasmids are available at Addgene for Orange CaMBI 110 (124094), Orange CaMBI 300 (124095), Blue CaMBI (124096), and Green CaMBI (124097). All other data from this study are available from the corresponding author upon request.
References
Clapham, D. E. Cell 131, 1047–1058 (2007).
Russell, J. T. Br. J. Pharmacol. 163, 1605–1625 (2011).
Tsien, R. Y., Ernst, L. & Waggoner, A. in Handbook Of Biological Confocal Microscopy (ed. Pawley, J.) 338–352 (Springer, 2006).
Chu, J. et al. Nat. Methods 11, 572–578 (2014).
Lin, M. Z. et al. Chem. Biol. 16, 1169–1179 (2009).
Zhao, H. et al. J. Biomed. Opt. 10, 41210 (2005).
Wang, A., Feng, J., Li, Y. & Zou, P. ACS Chem. Neurosci. 9, 639–650 (2018).
Martin, J. R. J. Neurogenet. 22, 285–307 (2008).
Bakayan, A., Domingo, B., Miyawaki, A. & Llopis, J. Pflugers Arch. 467, 2031–2042 (2015).
Shimomura, O., Kishi, Y. & Inouye, S. Biochem. J. 296, 549–551 (1993).
Hoshino, H., Nakajima, Y. & Ohmiya, Y. Nat. Methods 4, 637–639 (2007).
Saito, K. et al. Nat. Commun. 3, 1262 (2012).
Chu, J. et al. Nat. Biotechnol. 34, 760–767 (2016).
Suzuki, K. et al. Nat. Commun. 7, 13718 (2016).
Horikawa, K. et al. Nat. Methods 7, 729–732 (2010).
Chen, T. W. et al. Nature 499, 295–300 (2013).
Yang, J. et al. Nat. Commun. 7, 13268 (2016).
Reddy, R. et al. J. Biol. Chem. 270, 14340–14346 (1995).
Stanika, R. I., Villanueva, I., Kazanina, G., Andrews, S. B. & Pivovarova, N. B. J. Neurosci. 32, 6642–6650 (2012).
Gaspers, L. D., Pierobon, N. & Thomas, A. P. in Signaling Pathways in Liver Diseases (eds Dufour, J. F., Clavien, P. A., Trautwein, C. & Graf, R.) 211–221 (Springer, 2005).
Dupont, G., Combettes, L. & Leybaert, L. Int. Rev. Cytol. 261, 193–245 (2007).
Kuo, I. Y. & Ehrlich, B. E. Cold Spring Harb. Perspect. Biol. 7, a006023 (2015).
Penn, R. B. & Benovic, J. L. Proc. Am. Thorac. Soc. 5, 47–57 (2008).
Kono, M. et al. Nat. Commun. 8, 1163 (2017).
Takakura, H., Hattori, M., Takeuchi, M. & Ozawa, T. ACS Chem. Biol. 7, 901–910 (2012).
Kim, D. E., Chivian, D. & Baker, D. Nucleic Acids Res. 32, W526–W531 (2004).
Zhang, Y. BMC Bioinformatics 9, 40 (2008).
Tsien, R. & Pozzan, T. Methods Enzymol. 172, 230–262 (1989).
Wilkins, M. R. et al. Methods Mol. Biol. 112, 531–552 (1999).
Schindelin, J. et al. Nat. Methods 9, 676–682 (2012).
Bajar, B. T. et al. Sci. Rep. 6, 20889 (2016).
Lam, A. J. et al. Nat. Methods 9, 1005–1012 (2012).
Burridge, P. W. et al. Nat. Methods 11, 855–860 (2014).
Tasic, B. et al. Proc. Natl Acad. Sci. USA 108, 7902–7907 (2011).
Fiebig, T. et al. PLoS One 7, e31179 (2012).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. Behav. Res. Methods 39, 175–191 (2007).
Chuong, A. S. et al. Nat. Neurosci. 17, 1123–1129 (2014).
Horton, N. G. et al. Nat. Photon. 7, 205–209 (2013).
Podgorski, K. & Ranganathan, G. J. Neurophysiol. 116, 1012–1023 (2016).
Levin, R. A. et al. PLoS ONE 9, e97415 (2014).
Sarkar, S., Malekshah, O. M., Nomani, A., Patel, N. & Hatefi, A. Cancer Med. 7, 3630–3641 (2018).
Baklaushev, V. P. et al. Sci. Rep. 7, 7715 (2017).
Acknowledgements
We thank members of the Lin laboratory for assistance with experiments, and the Stanford Transgenic, Knockout, and Tumor Model Center for generating the Orange 110 CaMBI transgenic mice. This work was supported by an AHA Postdoctoral Fellowship (to N.K.), an AHA Innovation Grant 15IRG23290018 (to M.Z.L.), a Stanford Discovery Innovation Award (to M.Z.L. and Y.P.), NIH grants R01HL133272 (to J.C.W.), R01HL128170 (to J.C.W.), U01HL099776 (to J.C.W.), and U01NS090600 (to M.Z.L.); NIH fellowship F32HL119059 (to N.K.P.); and NIH Pioneer Award 5DP1GM111003 (to M.Z.L.).
Author information
Authors and Affiliations
Contributions
Y.O. developed CaMBIs, characterized CaMBI properties, performed cellular and animal experiments, performed analysis, and co-wrote the manuscript. Y.P. characterized CaMBI properties, generated trangenic mice, performed cellular and animal experiments, and performed analysis. J.C. and N.K. performed additional characterization of CaMBIs. H.W. assisted in culture of cardiomyocytes. N.K.P. and L.L. assisted with animal experiments. M.A.K. and J.C.W. provided training and advice. M.Z.L. designed experiments, performed analysis, provided training and advice, and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Supplementary Figures 1–8
Supplementary Video 1
Histamine-induced calcium oscillations in HeLa cells reported by Orange CaMBI 110. Time-lapse images of the cells in Supplementary Fig. 4a are shown.
Supplementary Video 2
Histamine-induced calcium oscillations in HeLa cells reported by Green CaMBI 110. Time-lapse images of the cells in Supplementary Fig. 4b are shown.
Supplementary Video 3
Spontaneous calcium oscillations in a cardiomyocyte reported by Orange CaMBI 110. Time-lapse images of the cells in Fig. 1d are shown.
Supplementary Video 4
Spontaneous calcium oscillations in neurons reported by Orange CaMBI 110. Time-lapse images of the cells in Fig. 1e are shown.
Supplementary Video 5
Spontaneous calcium oscillations in neurons reported by Orange CaMBI 300. Time-lapse images of the cells in Supplementary Fig. 4e are shown.
Supplementary Video 6
Noninvasive imaging of vasopressin-induced calcium oscillations in mouse liver by Orange CaMBI 110 with expression gated by viral-transduced cre. Time-lapse images of the mouse in Fig. 2 are shown.
Supplementary Video 7
Noninvasive imaging of vasopressin-induced calcium oscillations in mouse liver by Orange CaMBI 110 with expression gated by an albumin-cre transgene. Time-lapse images of the mouse in Supplementary Fig. 8 are shown.
Rights and permissions
About this article
Cite this article
Oh, Y., Park, Y., Cho, J.H. et al. An orange calcium-modulated bioluminescent indicator for non-invasive activity imaging. Nat Chem Biol 15, 433–436 (2019). https://doi.org/10.1038/s41589-019-0256-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0256-z
This article is cited by
-
In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter
Nature Communications (2023)
-
An optimized bioluminescent substrate for non-invasive imaging in the brain
Nature Chemical Biology (2023)
-
Neural engineering with photons as synaptic transmitters
Nature Methods (2023)
-
A luciferase prosubstrate and a red bioluminescent calcium indicator for imaging neuronal activity in mice
Nature Communications (2022)
-
A non-invasive system to monitor in vivo neural graft activity after spinal cord injury
Communications Biology (2022)