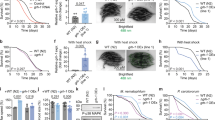
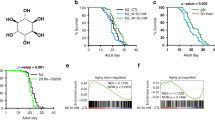
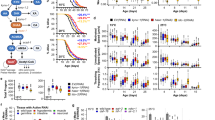
Abstract
Phenotypic screening has identified small-molecule modulators of aging, but the mechanism of compound action often remains opaque due to the complexities of mapping protein targets in whole organisms. Here, we combine a library of covalent inhibitors with activity-based protein profiling to coordinately discover bioactive compounds and protein targets that extend lifespan in Caenorhabditis elegans. We identify JZL184—an inhibitor of the mammalian endocannabinoid (eCB) hydrolase monoacylglycerol lipase (MAGL or MGLL)—as a potent inducer of longevity, a result that was initially perplexing as C. elegans does not possess an MAGL ortholog. We instead identify FAAH-4 as a principal target of JZL184 and show that this enzyme, despite lacking homology with MAGL, performs the equivalent metabolic function of degrading eCB-related monoacylglycerides in C. elegans. Small-molecule phenotypic screening thus illuminates pure pharmacological connections marking convergent metabolic functions in distantly related organisms, implicating the FAAH-4/monoacylglyceride pathway as a regulator of lifespan in C. elegans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper (and its supplementary information files) or from the corresponding author upon reasonable request.
References
Finkel, T. The metabolic regulation of aging. Nat. Med. 21, 1416–1423 (2015).
Imai, S. & Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 (2014).
Johnson, S. C., Rabinovitch, P. S. & Kaeberlein, M . mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R . A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Hamilton, B. et al. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 19, 1544–1555 (2005).
Garigan, D. et al. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 161, 1101–1112 (2002).
Evason, K., Huang, C., Yamben, I., Covey, D. F. & Kornfeld, K. Anticonvulsant medications extend worm life-span. Science 307, 258–262 (2005).
Petrascheck, M., Ye, X. & Buck, L. B. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556 (2007).
Bachovchin, D. A. et al. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc. Natl Acad. Sci. USA 107, 20941–20946 (2010).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Grüner, B. M. et al. An in vivo multiplexed small-molecule screening platform. Nat. Methods 13, 883–889 (2016).
Roberts, A. M. et al. Chemoproteomic screening of covalent ligands reveals UBA5 as a novel pancreatic cancer target. ACS Chem. Biol. 12, 899–904 (2017).
Hsu, K. L. et al. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 8, 999–1007 (2012).
Cognetta, A. B. et al. Selective N-hydroxyhydantoin carbamate inhibitors of mammalian serine hydrolases. Chem. Biol. 22, 928–937 (2015).
Liu, Y., Patricelli, M. P. & Cravatt, B. F. Activity-based protein profiling: the serine hydrolases. Proc. Natl Acad. Sci. USA 96, 14694–14699 (1999).
Niphakis, M. J. & Cravatt, B. F. Enzyme inhibitor discovery by activity-based protein profiling. Annu. Rev. Biochem. 83, 341–377 (2014).
Lucanic, M. et al. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 473, 226–229 (2011).
Lin, Y. H. et al. Diacylglycerol lipase regulates lifespan and oxidative stress response by inversely modulating TOR signaling in Drosophila and C. elegans. Aging Cell 13, 755–764 (2014).
Wang, M. C., O’Rourke, E. J. & Ruvkun, G. Fat metabolism links germline stem cells and longevity in C. elegans. Science 322, 957–960 (2008).
Folick, A. et al. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 347, 83–86 (2015).
Rangaraju, S. et al. Suppression of transcriptional drift extends C. elegans lifespan by postponing the onset of mortality. eLife 4, e08833 (2015).
Adibekian, A. et al. Click-generated triazole ureas as ultrapotent in vivo-active serine hydrolase inhibitors. Nat. Chem. Biol. 7, 469–478 (2011).
Chang, J. W., Cognetta, A. B. III, Niphakis, M. J. & Cravatt, B. F. Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition. ACS Chem. Biol. 8, 1590–1599 (2013).
Kamat, S. S. et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol. 11, 164–171 (2015).
Arantes-Oliveira, N., Apfeld, J., Dillin, A. & Kenyon, C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295, 502–505 (2002).
Long, J. Z. et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 5, 37–44 (2009).
Grabner, G. F., Zimmermann, R., Schicho, R. & Taschler, U. Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol. Ther. 175, 35–46 (2017).
Kathuria, S. et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 9, 76–81 (2003).
Shin, S. et al. Characterization of a novel Ser-cisSer-Lys catalytic triad in comparison with the classical Ser-His-Asp triad. J. Biol. Chem. 278, 24937–24943 (2003).
Cravatt, B. F. et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384, 83–87 (1996).
Giang, D. K. & Cravatt, B. F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl Acad. Sci. USA 94, 2238–2242 (1997).
Curnow, A. W. et al. Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl Acad. Sci. USA 94, 11819–11826 (1997).
Chang, J. W. et al. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem. Biol. 19, 579–588 (2012).
Dai, D. F., Chiao, Y. A., Marcinek, D. J., Szeto, H. H. & Rabinovitch, P. S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 3, 6 (2014).
Greer, E. L. & Brunet, A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127 (2009).
Altenhoff, A. M. et al. The OMA orthology database in 2018: retrieving evolutionary relationships among all domains of life through richer web and programmatic interfaces. Nucleic Acids Res. 46, D477–D485 (2017).
Dolinski, K. & Botstein, D. Orthology and functional conservation in eukaryotes. Annu. Rev. Genet. 41, 465–507 (2007).
Omelchenko, M. V., Galperin, M. Y., Wolf, Y. I. & Koonin, E. V. Non-homologous isofunctional enzymes: a systematic analysis of alternative solutions in enzyme evolution. Biol. Direct 5, 31 (2010).
Bandyopadhyay, S., Sharan, R. & Ideker, T. Systematic identification of functional orthologs based on protein network comparison. Genome Res. 16, 428–435 (2006).
Kurnasov, O. et al. NAD biosynthesis: identification of the tryptophan to quinolinate pathway in bacteria. Chem. Biol. 10, 1195–1204 (2003).
Martell, J. et al. Global cysteine-reactivity profiling during impaired insulin/IGF-1 signaling in C. elegans identifies uncharacterized mediators of longevity. Cell Chem. Biol. 23, 955–966 (2016).
Han, S. et al. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 544, 185–190 (2017).
O’Rourke, E. J., Kuballa, P., Xavier, R. & Ruvkun, G. ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 27, 429–440 (2013).
Shmookler Reis, R. J. et al. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging (Albany NY) 3, 125–147 (2011).
Oakes, M. D., Law, W. J., Clark, T., Bamber, B. A. & Komuniecki, R. Cannabinoids activate monoaminergic signaling to modulate key C. elegans behaviors. J. Neurosci. 37, 2859–2869 (2017).
Ogasawara, D. et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc. Natl Acad. Sci. USA 113, 26–33 (2016).
Fagan, S. G. & Campbell, V. A. The influence of cannabinoids on generic traits of neurodegeneration. Br. J. Pharmacol. 171, 1347–1360 (2014).
Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Phil. Trans. R. Soc. Lond. B 367, 3326–3341 (2012).
Piyanova, A. et al. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech. Ageing Dev. 150, 55–64 (2015).
Paix, A., Folkmann, A., Rasoloson, D. & Seydoux, G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics 201, 47–54 (2015).
Rangaraju, S., Solis, G. M. & Petrascheck, M. High-throughput small-molecule screening in Caenorhabditis elegans. Methods Mol. Biol. 1263, 139–155 (2015).
Hulce, J. J., Cognetta, A. B., Niphakis, M. J., Tully, S. E. & Cravatt, B. F. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods 10, 259–264 (2013).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise huisgen cycloaddition process: copper(i)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew.Chem. Int. Edn Engl. 41, 2596–2599 (2002).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Solis, G. M. et al. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. eLife 7, e40314 (2018).
Jessani, N. et al. A streamlined platform for high-content functional proteomics of primary human specimens. Nat. Methods 2, 691–697 (2005).
Gomez-Amaro, R. L. et al. Measuring food intake and nutrient absorption in Caenorhabditis elegans. Genetics 200, 443–454 (2015).
Bar-Peled, L. et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106 (2013).
Patricelli, M. P., Giang, D. K., Stamp, L. M. & Burbaum, J. J. Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics 1, 1067–1071 (2001).
Acknowledgements
We are grateful to M. Hansen, J. Chang, X. She, and G. Solis for discussions and technical expertise in C. elegans, D. Sabatini for the pRK5 vector, and the Caenorhabditis Genetics Center for strains. This work was supported by the NIH (DA033760, CA215249, GM074215), the Damon Runyon Cancer Research Foundation, and Abide Therapeutics.
Author information
Authors and Affiliations
Contributions
A.L.C., L.B.-P., and B.F.C. conceived the project and wrote the paper. A.L.C., M.P., L.B.-P., and B.F.C. designed the experiments. A.L.C., M.P., and L.B.-P. developed the methods. A.L.C. performed the experiments and analyzed data. K.M.L. and G.M.S. generated the putative SH list and nonredundant C. elegans database. A.L.C. and K.M.L. analyzed chemical proteomic data. A.L.C., K.M.L., D.O., A.B.C., and W.H.P. designed and synthesized compounds. A.L.C., P.L.-G. and A.T. generated CRISPR–Cas9 mediated strains and A.D. and M.P. provided the facilities. P. L.-G. and A.L.C. backcrossed and sequenced CRISPR–Cas9-mediated strains. M.P. and A.T. assisted with lifespan experiments and RNA-seq analysis. A.L.C. and G.M.S. generated dendrograms. K.M.L., P.L.-G., D.O., W.H.P., A.D., and M.P. edited the paper.
Corresponding authors
Ethics declarations
Competing interests
B.F.C. is a founder and advisor to Abide Therapeutics, a biotechnology company interested in developing serine hydrolase inhibitors and therapeutics.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–6, Supplementary Figures 1–12
Supplementary Note 1
Synthetic methods
Supplementary Dataset 1
List of predicted C. elegans SHs, FP-enriched SHs, and gene expression data, related to Fig. 1.
Supplementary Dataset 2
Lifespan data.
Supplementary Dataset 3
Proteomic data, related to Figs. 3 and 4 and Supplementary Figure 7.
Supplementary Dataset 4
Proteomic data, related to Figs. 3 and 4 and Supplementary Table 7.
Supplementary Dataset 5
Proteomic data, related to Fig. 3.
Rights and permissions
About this article
Cite this article
Chen, A.L., Lum, K.M., Lara-Gonzalez, P. et al. Pharmacological convergence reveals a lipid pathway that regulates C. elegans lifespan. Nat Chem Biol 15, 453–462 (2019). https://doi.org/10.1038/s41589-019-0243-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0243-4
This article is cited by
-
Two legume fatty acid amide hydrolase isoforms with distinct preferences for microbial- and plant-derived acylamides
Scientific Reports (2023)
-
Lipid droplets and peroxisomes are co-regulated to drive lifespan extension in response to mono-unsaturated fatty acids
Nature Cell Biology (2023)
-
Lipid metabolism and ageing in Caenorhabditis elegans: a complex interplay
Biogerontology (2022)
-
Scaffold and organism hopping with chemical probes
Nature Chemical Biology (2019)