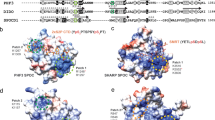
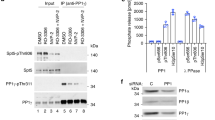
Abstract
Phosphorylation of the carboxyl-terminal domain (CTD) of the largest subunit of RNA polymerase II (Pol II) governs stage-specific interactions with different cellular machines. The CTD consists of Y1S2P3T4S5P6S7 heptad repeats and sequential phosphorylations of Ser7, Ser5 and Ser2 occur universally at Pol II-transcribed genes. Phosphorylation of Thr4, however, appears to selectively modulate transcription of specific classes of genes. Here, we identify ten new Thr4 kinases from different kinase structural groups. Irreversible chemical inhibition of the most active Thr4 kinase, Hrr25, reveals a novel role for this kinase in transcription termination of specific class of noncoding snoRNA genes. Genome-wide profiles of Hrr25 reveal a selective enrichment at 3ʹ regions of noncoding genes that display termination defects. Importantly, phospho-Thr4 marks placed by Hrr25 are recognized by Rtt103, a key component of the termination machinery. Our results suggest that these uncommon CTD kinases place phospho-Thr4 marks to regulate expression of targeted genes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All ChIP and RNA-seq data are available at the Gene Expression Omnibus under the accession number: GSE107166.
References
Corden, J. L., Cadena, D. L., Ahearn, J. M. Jr. & Dahmus, M. E. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc. Natl Acad. Sci. USA 82, 7934–7938 (1985).
Zhang, D. W., Rodríguez-Molina, J. B., Tietjen, J. R., Nemec, C. M. & Ansari, A. Z. Emerging views on the CTD code. Genet. Res. Int. 2012, 347214 (2012).
Eick, D. & Geyer, M. The RNA polymerase II carboxy-terminal domain (CTD) code. Chem. Rev. 113, 8456–8490 (2013).
Corden, J. L. RNA polymerase II C-terminal domain: tethering transcription to transcript and template. Chem. Rev. 113, 8423–8455 (2013).
Phatnani, H. P. & Greenleaf, A. L. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922–2936 (2006).
Kornberg, R. D. Mediator and the mechanism of transcriptional activation. Trends. Biochem. Sci. 30, 235–239 (2005).
Feaver, W. J., Svejstrup, J. Q., Henry, N. L. & Kornberg, R. D. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell 79, 1103–1109 (1994).
Akhtar, M. S. et al. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol. Cell 34, 387–393 (2009).
Cho, E. J., Takagi, T., Moore, C. R. & Buratowski, S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 11, 3319–3326 (1997).
Bharati, A. P. et al. The mRNA capping enzyme of Saccharomyces cerevisiae has dual specificity to interact with CTD of RNA polymerase II. Sci. Rep. 6, 31294 (2016).
Qiu, H., Hu, C. & Hinnebusch, A. G. Phosphorylation of the Pol II CTD by KIN28 enhances BUR1/BUR2 recruitment and Ser2 CTD phosphorylation near promoters. Mol. Cell 33, 752–762 (2009).
Murray, S., Udupa, R., Yao, S., Hartzog, G. & Prelich, G. Phosphorylation of the RNA polymerase II carboxy-terminal domain by the Bur1 cyclin-dependent kinase. Mol. Cell. Biol. 21, 4089–4096 (2001).
Jones, J. C. et al. C-terminal repeat domain kinase I phosphorylates Ser2 and Ser5 of RNA polymerase II C-terminal domain repeats. J. Biol. Chem. 279, 24957–24964 (2004).
Hintermair, C. et al. Threonine-4 of mammalian RNA polymerase II CTD is targeted by Polo-like kinase 3 and required for transcriptional elongation. EMBO J. 31, 2784–2797 (2012).
Hsin, J. P., Sheth, A. & Manley, J. L. RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3′ end processing. Science 334, 683–686 (2011).
Rosonina, E. et al. Threonine-4 of the budding yeast RNAP II CTD couples transcription with Htz1-mediated chromatin remodeling. Proc. Natl Acad. Sci. USA 111, 11924–11931 (2014).
Schwer, B., Bitton, D. A., Sanchez, A. M., Bähler, J. & Shuman, S. Individual letters of the RNA polymerase II CTD code govern distinct gene expression programs in fission yeast. Proc. Natl Acad. Sci. USA 111, 4185–4190 (2014).
Harlen, K. M. et al. Comprehensive RNA polymerase II interactomes reveal distinct and varied roles for each phospho-CTD residue. Cell Rep. 15, 2147–2158 (2016).
Nemec, C. M. et al. Different phosphoisoforms of RNA polymerase II engage the Rtt103 termination factor in a structurally analogous manner. Proc. Natl Acad. Sci. USA 114, E3944–E3953 (2017).
Hintermair, C. et al. Specific threonine-4 phosphorylation and function of RNA polymerase II CTD during M phase progression. Sci. Rep. 6, 27401 (2016).
Tietjen, J. R. et al. Chemical-genomic dissection of the CTD code. Nat. Struct. Mol. Biol. 17, 1154–1161 (2010).
Liu, Y. et al. Phosphorylation of the transcription elongation factor Spt5 by yeast Bur1 kinase stimulates recruitment of the PAF complex. Mol. Cell. Biol. 29, 4852–4863 (2009).
Coudreuse, D. et al. A gene-specific requirement of RNA polymerase II CTD phosphorylation for sexual differentiation in S. pombe. Curr. Biol. 20, 1053–1064 (2010).
Rodríguez-Molina, J. B., Tseng, S. C., Simonett, S. P., Taunton, J. & Ansari, A. Z. Engineered covalent inactivation of TFIIH-kinase reveals an elongation checkpoint and results in widespread mRNA stabilization. Mol. Cell 63, 433–444 (2016).
Bishop, A. C. et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 (2000).
Hsin, J. P., Xiang, K. & Manley, J. L. Function and control of RNA polymerase II C-terminal domain phosphorylation in vertebrate transcription and RNA processing. Mol. Cell. Biol. 34, 2488–2498 (2014).
Chymkowitch, P. et al. Cdc28 kinase activity regulates the basal transcription machinery at a subset of genes. Proc. Natl Acad. Sci. USA 109, 10450–10455 (2012).
Keogh, M. C., Podolny, V. & Buratowski, S. Bur1 kinase is required for efficient transcription elongation by RNA polymerase II. Mol. Cell. Biol. 23, 7005–7018 (2003).
Phatnani, H. P., Jones, J. C. & Greenleaf, A. L. Expanding the functional repertoire of CTD kinase I and RNA polymerase II: novel phosphoCTD-associating proteins in the yeast proteome. Biochemistry 43, 15702–15719 (2004).
Hoekstra, M. F. et al. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science 253, 1031–1034 (1991).
Ho, Y., Mason, S., Kobayashi, R., Hoekstra, M. & Andrews, B. Role of the casein kinase I isoform, Hrr25, and the cell cycle-regulatory transcription factor, SBF, in the transcriptional response to DNA damage in Saccharomyces cerevisiae . Proc. Natl Acad. Sci. USA 94, 581–586 (1997).
Schäfer, T. et al. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature 441, 651–655 (2006).
Petronczki, M. et al. Monopolar attachment of sister kinetochores at meiosis I requires casein kinase 1. Cell 126, 1049–1064 (2006).
Pfaffenwimmer, T. et al. Hrr25 kinase promotes selective autophagy by phosphorylating the cargo receptor Atg19. EMBO Rep. 15, 862–870 (2014).
Measday, V. et al. A family of cyclin-like proteins that interact with the Pho85 cyclin-dependent kinase. Mol. Cell. Biol. 17, 1212–1223 (1997).
Mitchell, A. P. & Bowdish, K. S. Selection for early meiotic mutants in yeast. Genetics 131, 65–72 (1992).
Yabuki, Y. et al. Glycogen synthase kinase-3 is involved in regulation of ribosome biogenesis in yeast. Biosci. Biotechnol. Biochem. 78, 800–805 (2014).
Posas, F. & Saito, H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17, 1385–1394 (1998).
Sánchez-Piris, M. et al. The serine/threonine kinase Cmk2 is required for oxidative stress response in fission yeast. J. Biol. Chem. 277, 17722–17727 (2002).
Posas, F. et al. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86, 865–875 (1996).
Chasman, D. et al. Pathway connectivity and signaling coordination in the yeast stress-activated signaling network. Mol. Syst. Biol. 10, 759 (2014).
Ferreira-Cerca, S. et al. ATPase-dependent role of the atypical kinase Rio2 on the evolving pre-40S ribosomal subunit. Nat. Struct. Mol. Biol. 19, 1316–1323 (2012).
Liu, Y. et al. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol. Cell. Biol. 24, 1721–1735 (2004).
Garcia, B., Stollar, E. J. & Davidson, A. R. The importance of conserved features of yeast actin-binding protein 1 (Abp1p): the conditional nature of essentiality. Genetics 191, 1199–1211 (2012).
Kanin, E. I. et al. Chemical inhibition of the TFIIH-associated kinase Cdk7/Kin28 does not impair global mRNA synthesis. Proc. Natl Acad. Sci. USA 104, 5812–5817 (2007).
Gasch, A. P. et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 (2000).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Flotow, H. et al. Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem. 265, 14264–14269 (1990).
Ansari, A. Z., Ogirala, A. & Ptashne, M. Transcriptional activating regions target attached substrates to a cyclin-dependent kinase. Proc. Natl Acad. Sci. USA 102, 2346–2349 (2005).
Churchman, L. S. & Weissman, J. S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–373 (2011).
Schrödinger, L. The PyMOL Molecular Graphics System, Version 1.3. (2010).
Gasch, A. P. in Methods in Enzymol ogy Vol. 350 (eds. Guthrie, C. & Fink, G. R.) 393–414 (Academic Press, 2002).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome. Biol. 11, R106 (2010).
Ramírez, F., Dündar, F., Diehl, S., Grüning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014).
Chanfreau, G., Rotondo, G., Legrain, P. & Jacquier, A. Processing of a dicistronic small nucleolar RNA precursor by the RNA endonuclease Rnt1. EMBO J. 17, 3726–3737 (1998).
Kim, M. et al. Distinct pathways for snoRNA and mRNA termination. Mol. Cell 24, 723–734 (2006).
Acknowledgements
We thank K. Moeller for preliminary data, and Professors J. Corden (Johns Hopkins School of Medicine), E. Hurt (University of Heidelberg), B. Andrews (University of Toronto), A. Greenleaf (Duke University), S. Hahn (Fred Hutchinson Cancer Research, M. Stark (University of Dundee), and M. Webb (The Francis Crick Institute) for plasmids and strains. We thank members of the Ansari lab, especially J. Rodríguez-Molina, for thoughtful comments, suggestions, and advice. This work was funded by NSF MCB1413547 and NIH GM117362 to A.Z.A., NIH R01 GM083989 to A.P.G., Deutsche Forschungsgemeinschaft, SFB1064, Chromatin Dynamics to DE, The Department of Biotechnology, Government of India (GAP0103) to M.S.A. A.A. and M.F.A. are supported by an Indo-US postdoctoral fellowship from SERB, Government of India.
Author information
Authors and Affiliations
Contributions
C.M.N., A.K.S., and K.J.R. purified yeast kinases and performed in vitro kinase assays. M.S.A. guided A.K.S. and provided in vitro kinase data. C.M.N. tested analog-sensitive and irreversibly sensitive kinases in vivo. K.S. and M.F.A. performed inhibition time course and collected IC50 data. R.K.K. worked with K.S. to generate the Kin28is strain. A.A. docked CMK and 3-MB-PP1 into structures of Hrr25. C.M.N. purified RNA and performed northern blot analysis. Y.-H.H. and A.P.G. performed analysis of hrr25as RNA-seq. S.C.T. prepared hrr25is RNA-seq libraries, and S.C.T. and C.M.N. analyzed hrr25is RNA-seq data. ChIP-chip experiments were performed by C.M.N., and ChIP-qPCR was performed by A.K.S. C.M.N. and K.S. performed binding experiments. C.H. and D.E. provided antibodies and shared data, protocols, and insights to facilitate this project. C.M.N. and A.Z.A. conceived the project and wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
A.Z.A. is the sole member of VistaMotif, LLC and founder of the educational US nonprofit WINStep Forward.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–3
Rights and permissions
About this article
Cite this article
Nemec, C.M., Singh, A.K., Ali, A. et al. Noncanonical CTD kinases regulate RNA polymerase II in a gene-class-specific manner. Nat Chem Biol 15, 123–131 (2019). https://doi.org/10.1038/s41589-018-0194-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0194-1
This article is cited by
-
Prognostic value and immunological role of PD-L1 gene in pan-cancer
BMC Cancer (2024)
-
Expression profiles and function prediction of tRNA-derived fragments in glioma
BMC Cancer (2023)
-
Dissecting the Pol II transcription cycle and derailing cancer with CDK inhibitors
Nature Chemical Biology (2020)
-
Conducting the CTD orchestra
Nature Chemical Biology (2019)