Abstract
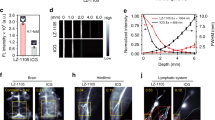
The spatiotemporal generation of nitric oxide (NO), a versatile endogenous messenger, is precisely controlled. Despite its therapeutic potential for a wide range of diseases, NO-based therapies are limited clinically due to a lack of effective strategies for precisely delivering NO to a specific site. In the present study, we developed a novel NO delivery system via modification of an enzyme–prodrug pair of galactosidase–galactosyl-NONOate using a ‘bump-and-hole’ strategy. Precise delivery to targeted tissues was clearly demonstrated by an in vivo near-infrared imaging assay. The therapeutic potential was evaluated in both rat hindlimb ischemia and mouse acute kidney injury models. Targeted delivery of NO clearly enhanced its therapeutic efficacy in tissue repair and function recovery and abolished side effects due to the systemic release of NO. The developed protocol holds broad applicability in the targeted delivery of important gaseous signaling molecules and offers a potent tool for the investigation of relevant molecular mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and supplementary information files and from the corresponding authors upon reasonable request.
References
Förstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837, 837a–837d (2012).
SoRelle, R. Nobel prize awarded to scientists for nitric oxide discoveries. Circulation 98, 2365–2366 (1998).
de Carvalho, M. P., Weich, H. & Abraham, W.-R. Macrocyclic trichothecenes as antifungal and anticancer compounds. Curr. Med. Chem. 23, 23–35 (2016).
Miller, M. R. & Megson, I. L. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 151, 305–321 (2007).
Baek, S. H. et al. Augmentation of intrapericardial nitric oxide level by a prolonged-release nitric oxide donor reduces luminal narrowing after porcine coronary angioplasty. Circulation 105, 2779–2784 (2002).
Kapadia, M. R. et al. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J. Vasc. Surg. 47, 173–182 (2008).
Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2, 907–916 (2001).
Mazzone, M. & Carmeliet, P. Drug discovery: a lifeline for suffocating tissues. Nature 453, 1194–1195 (2008).
Kashiwagi, S. et al. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J. Clin. Invest. 115, 1816–1827 (2005).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug. Discov. 7, 156–167 (2008).
Hrabie, J. A. & Keefer, L. K. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem. Rev. 102, 1135–1154 (2002).
Wu, X., Tang, X., Xian, M. & Wang, P. G. Glycosylated diazeniumdiolates: a novel class of enzyme-activated nitric oxide donors. Tetrahedr. Lett. 42, 3779–3782 (2001).
Zhao, Q. et al. Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis. Biomaterials. 34, 8450–8458 (2013).
Wang, Z. et al. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Control Release 210, 179–188 (2015).
Chandrawati, R. et al. Localized and controlled delivery of nitric oxide to the conventional outflow pathway via enzyme biocatalysis: toward therapy for glaucoma. Adv. Mater. 29, 1604932 (2017).
Chandrawati, R. et al. Enzyme prodrug therapy engineered into electrospun fibers with embedded liposomes for controlled, localized synthesis of therapeutics. Adv. Healthc. Mater. 6, 1700385 (2017).
Lundberg, J. O., Gladwin, M. T. & Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug. Discov. 14, 623–641 (2015).
Springer, C. J. & Niculescu-Duvaz, I. Antibody-directed enzyme prodrug therapy (ADEPT): a review. Adv. Drug Deliv. Rev. 26, 151–172 (1997).
Städler, B. & Zelikin, A. N. Enzyme prodrug therapies and therapeutic enzymes. Adv. Drug Deliv. Rev. 118, 1 (2017).
Walther, R., Rautio, J. & Zelikin, A. N. Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv. Drug Deliv. Rev. 118, 65–77 (2017).
Shah, K., Liu, Y., Deirmengian, C. & Shokat, K. M. Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc. Natl Acad. Sci. USA 94, 3565–3570 (1997).
Bishop, A. C. et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 (2000).
Baud, M. G. J. et al. Chemical biology. A bump-and-hole approach to engineer controlled selectivity of BET bromodomain chemical probes. Science 346, 638–641 (2014).
Baud, M. G., Lin-Shiao, E., Zengerle, M., Tallant, C. & Ciulli, A. New synthetic routes to triazolo-benzodiazepine analogues: expanding the scope of the bump-and-hole approach for selective bromo and extra-terminal (BET) bromodomain inhibition. J. Med. Chem. 59, 1492–1500 (2016).
Islam, K. et al. Defining efficient enzyme-cofactor pairs for bioorthogonal profiling of protein methylation. Proc. Natl Acad. Sci. USA 110, 16778–16783 (2013).
Cai, T. B., Lu, D., Landerholm, M. & Wang, P. G. Sialated diazeniumdiolate: a new sialidase-activated nitric oxide donor. Org. Lett. 6, 4203–4205 (2004).
Nagano, T. & Yoshimura, T. Bioimaging of nitric oxide. Chem. Rev. 102, 1235–1270 (2002).
Sabbisetti, V. S. et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 25, 2177–2186 (2014).
Portilla, D. et al. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 69, 2194–2204 (2006).
Vaziri, N. D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 12, 37–47 (2016).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Sun, Y. B. Y., Qu, X., Caruana, G. & Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 92, 102–107 (2016).
Liu, Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 69, 213–217 (2006).
Feng, G. et al. IGF-1 C domain-modified hydrogel enhances cell Therapy for AKI. J. Am. Soc. Nephrol. 27, 2357–2369 (2016).
Chiazza, F. et al. A nitric oxide-donor furoxan moiety improves the efficacy of edaravone against early renal dysfunction and injury evoked by ischemia/reperfusion. Oxid. Med. Cell Longev. 2015, 804659 (2015).
Yuen, D. A. et al. eNOS deficiency predisposes podocytes to injury in diabetes. J. Am. Soc. Nephrol. 23, 1810–1823 (2012).
Cheng, H. et al. Synthesis and enzyme-specific activation of carbohydrate-geldanamycin conjugates with potent anticancer activity. J. Med. Chem. 48, 645–652 (2005).
Bolam, D. N. et al. The crystal structure of two macrolide glycosyltransferases provides a blueprint for host cell antibiotic immunity. Proc. Natl Acad. Sci. USA 104, 5336–5341 (2007).
Ramakrishnan, B. & Qasba, P. K. Structure-based design of β1,4-galactosyltransferase I (β4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity: point mutation broadens β4Gal-T1 donor specificity. J. Biol. Chem. 277, 20833–20839 (2002).
Uchida, N. et al. Chemical hijacking of auxin signaling with an engineered auxin-TIR1 pair. Nat. Chem. Biol. 14, 299–305 (2018).
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Cao, R. et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 9, 604–613 (2003).
Place, E. S., Evans, N. D. & Stevens, M. M. Complexity in biomaterials for tissue engineering. Nat. Mater. 8, 457–470 (2009).
Cooke, J. P. NO and angiogenesis. Atheroscler. Suppl. 4, 53–60 (2003).
Ziche, M. et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Invest. 94, 2036–2044 (1994).
Jain, R. K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Khambata, R. S. et al. Antiinflammatory actions of inorganic nitrate stabilize the atherosclerotic plaque. Proc. Natl Acad. Sci. USA 114, E550–E559 (2017).
Liu, V. W. & Huang, P. L. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 77, 19–29 (2008).
Berg, J. D. & Fiksdal, L. Rapid detection of total and fecal coliforms in water by enzymatic hydrolysis of 4-methylumbelliferone-β-d-galactoside. Appl. Environ. Microbiol. 54, 2118–2122 (1988).
Huber, R. E., Hakda, S., Cheng, C., Cupples, C. G. & Edwards, R. A. Trp-999 of β-galactosidase (Escherichia coli) is a key residue for binding, catalysis, and synthesis of allolactose, the natural lac operon inducer. Biochemistry 42, 1796–1803 (2003).
Shinobu, L. A., Jones, S. G. & Jones, M. M. Sodium N-methyl-d-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol. Toxicol. (Copenh.) 54, 189–194 (1984).
Ricles, L. M. et al. Therapeutic assessment of mesenchymal stem cells delivered within a PEGylated fibrin gel following an ischemic injury. Biomaterials. 102, 9–19 (2016).
Du, W. et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials. 133, 70–81 (2017).
Kinnaird, T. et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109, 1543–1549 (2004).
Acknowledgements
We thank J. Xiao at Shanghai University for kindly donating eNOS-knockout mice. This study was supported by National Natural Science Foundation of China (Nos. 81522023 to Q.Z., 81830060 to D.K., 81603064 to J.H., 81871500 to Q.Z., and 91639113 to Q.Z.) and National Key R&D Program of China (2017YFC1103501 to D.K. and Q.Z.).
Author information
Authors and Affiliations
Contributions
J.S. and J.C. conceived the original concept and initiated this project. Q.Z. designed the experiment and supervised the entire project. Y.F. performed the expression of enzymes under the supervision of J.C. J.H. carried out the synthesis of all compounds and fluorescent probes. G.F. established ischemia animal models. Y.P. and D.Z. performed the in vivo evaluation and analyzed data under the supervision of Q.Z. J.H. and H.W. performed the in vitro releasing test of NO. Y.W. carried out the RT-PCR assay. T.Z. performed micro-CT analysis under the supervision of Y.Z. Y.L. helped with EPR analysis. K.Q., Y.C., and Q.Y. helped with data collection. P.G.W. and D.K. provided critical feedback and helped in review of the article. Q.Z., J.C., and J.H. wrote the paper with input from other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Figures 1–17
Supplementary Note 1
Synthetic Procedures
Rights and permissions
About this article
Cite this article
Hou, J., Pan, Y., Zhu, D. et al. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme–prodrug pair. Nat Chem Biol 15, 151–160 (2019). https://doi.org/10.1038/s41589-018-0190-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0190-5
This article is cited by
-
Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles
Nature Communications (2023)
-
A self-activated NO-releasing hydrogel depot for photothermal enhanced sterilization
Nano Research (2023)
-
Protective effect of platinum nano-antioxidant and nitric oxide against hepatic ischemia-reperfusion injury
Nature Communications (2022)
-
Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery
Nature Communications (2021)
-
Tumor Microenvironment Cascade-Responsive Nanodrug with Self-Targeting Activation and ROS Regeneration for Synergistic Oxidation-Chemotherapy
Nano-Micro Letters (2020)