Abstract
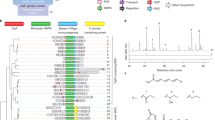
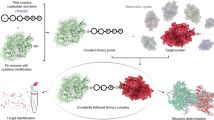
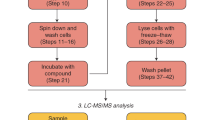
The nucleotide ppGpp is a highly conserved regulatory molecule in bacteria that helps tune growth rate to nutrient availability. Despite decades of study, how ppGpp regulates growth remains poorly understood. Here, we developed and validated a capture-compound mass spectrometry approach that identified >50 putative ppGpp targets in Escherichia coli. These targets control many key cellular processes and include 13 enzymes required for nucleotide synthesis. We demonstrated that ppGpp inhibits the de novo synthesis of all purine nucleotides by directly targeting the enzyme PurF. By solving a structure of PurF bound to ppGpp, we designed a mutation that ablates ppGpp-based regulation, leading to dysregulation of purine-nucleotide synthesis following ppGpp accumulation. Collectively, our results provide new insights into ppGpp-based growth control and a nearly comprehensive set of targets for future exploration. The capture compounds developed should also enable the rapid identification of ppGpp targets in any species, including pathogens.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Structural data for the PurF–ppGpp complex used to generate Figs. 4d–f, 5a and Supplementary Fig. 4b–d have been deposited in the Protein Data Bank under accession number PDB 6CZF. Raw proteomic LC–MS2 data as sources of Table 1, Fig. 2c and the Supplementary Dataset have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository under dataset identifier PXD010402. All other data generated or analyzed during this study are included in this published article (and its Supplementary Information files) or are available from the corresponding author on reasonable request.
References
Atkinson, G. C., Tenson, T. & Hauryliuk, V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6, e23479 (2011).
Potrykus, K., Murphy, H., Philippe, N. & Cashel, M. ppGpp is the major source of growth rate control in E. coli. Environ. Microbiol. 13, 563–575 (2011).
Hauryliuk, V., Atkinson, G. C., Murakami, K. S., Tenson, T. & Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 13, 298–309 (2015).
Liu, K., Bittner, A. N. & Wang, J. D. Diversity in (p)ppGpp metabolism and effectors. Curr. Opin. Microbiol. 24, 72–79 (2015).
Haseltine, W. A. & Block, R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl Acad. Sci. USA 70, 1564–1568 (1973).
Sarubbi, E. et al. Characterization of the spoT gene of Escherichia coli. J. Biol. Chem. 264, 15074–15082 (1989).
Mechold, U., Potrykus, K., Murphy, H., Murakami, K. S. & Cashel, M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. 41, 6175–6189 (2013).
Kanjee, U., Ogata, K. & Houry, W. A. Direct binding targets of the stringent response alarmone (p)ppGpp. Mol. Microbiol. 85, 1029–1043 (2012).
Potrykus, K. & Cashel, M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 (2008).
Dalebroux, Z. D. & Swanson, M. S. ppGpp: magic beyond RNA polymerase. Nat. Rev. Microbiol. 10, 203–212 (2012).
Ross, W. et al. ppGpp binding to a site at the RNAP-DksA interface accounts for its dramatic effects on transcription initiation during the stringent response. Mol. Cell 62, 811–823 (2016).
Traxler, M. F. et al. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 68, 1128–1148 (2008).
Zhang, Y., Zbornikova, E., Rejman, D. & Gerdes, K. Novel (p)ppGpp binding and metabolizing proteins of Escherichia coli. mBio 9, e02188–17 (2018).
Corrigan, R. M., Bellows, L. E., Wood, A. & Gründling, A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc. Natl Acad. Sci. USA 113, E1710–E1719 (2016).
Schreiber, G. et al. Overexpression of the relA gene in Escherichia coli. J. Biol. Chem. 266, 3760–3767 (1991).
Köster, H. et al. Capture compound mass spectrometry: a technology for the investigation of small molecule protein interactions. Assay. Drug. Dev. Technol. 5, 381–390 (2007).
Steinchen, W. Catalytic mechanism and allosteric regulation of an oligomeric (p)ppGpp synthetase by an alarmone. Proc. Natl Acad. Sci. USA 112, 13348–13353 (2015).
Mann, M. Functional and quantitative proteomics using SILAC. Nat. Rev. Mol. Cell Biol. 7, 952–958 (2006).
Li, G. W., Burkhardt, D., Gross, C. & Weissman, J. S. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157, 624–635 (2014).
Verstraeten, N., Fauvart, M., Versées, W. & Michiels, J. The universally conserved prokaryotic GTPases. Microbiol. Mol. Biol. Rev. 75, 507–542 (2011).
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Cashel, M. Regulation of bacterial ppGpp and pppGpp. Annu. Rev. Microbiol. 29, 301–318 (1975).
Messenger, L. J. & Zalkin, H. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli: purification and properties. J. Biol. Chem. 254, 3382–3392 (1979).
Krahn, J. M. et al. Coupled formation of an amidotransferase interdomain ammonia channel and a phosphoribosyltransferase active site. Biochemistry 36, 11061–11068 (1997).
Jensen, K. F., Dandanell, G., Hove-Jensen, B. & WillemoËs, M. Nucleotides, nucleosides, and nucleobases. Ecosal Plus 3, (2008).
Hove-Jensen, B., Harlow, K. W., King, C. J. & Switzer, R. L. Phosphoribosylpyrophosphate synthetase of Escherichia coli: properties of the purified enzyme and primary structure of the prs gene. J. Biol. Chem. 261, 6765–6771 (1986).
Milon, P. et al. The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc. Natl Acad. Sci. USA 103, 13962–13967 (2006).
Rojas, A. M., Ehrenberg, M., Andersson, S. G. E. & Kurland, C. G. ppGpp inhibition of elongation factors Tu, G and Ts during polypeptide synthesis. Mol. Gen. Genet. 197, 36–45 (1984).
Kriel, A. et al. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol. Cell 48, 231–241 (2012).
Liu, K. et al. Molecular mechanism and evolution of guanylate kinase regulation by (p)ppGpp. Mol. Cell 57, 735–749 (2015).
Krásný, L. & Gourse, R.L. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 23, 4473–4483 (2004).
Hochstadt-Ozer, J. & Cashel, M. The regulation of purine utilization in bacteria. V. Inhibition of purine phosphoribosyltransferase activities and purine uptake in isolated membrane vesicles by guanosine tetraphosphate. J. Biol. Chem. 247, 7067–7072 (1972).
Stevens, A. J. et al. Design of a split intein with exceptional protein splicing activity. J. Am. Chem. Soc. 138, 2162–2165 (2016).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Dai, P. et al. Salt effect accelerates site-selective cysteine bioconjugation. ACS Cent. Sci. 2, 637–646 (2016).
Park, J. O. et al. Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat. Chem. Biol. 12, 482–489 (2016).
Wang, B., Zhao, A., Novick, R. P. & Muir, T. W. Key driving forces in the biosynthesis of autoinducing peptides required for staphylococcal virulence. Proc. Natl Acad. Sci. USA 112, 10679–10684 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Muchmore, C. R., Krahn, J. M., Kim, J. H., Zalkin, H. & Smith, J. L. Crystal structure of glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Protein Sci. 7, 39–51 (1998).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Acknowledgements
We thank M. Guo, J. Kraemer, and P. Culviner for comments on the manuscript. We thank T. Muir (Princeton University) and S. Lovett (Brandeis University) for providing protein-expression vectors. This research made use of the Pilatus detector (RR029205) at NE-CAT beamline 24-IDC (GM103403) of the Advanced Photon Source (DE-AC02-06CH11357). We thank members of the Drennan laboratory for collecting the diffraction data at APS. We thank the Koch Institute Swanson Biotechnology Center (Biopolymer and Proteomic Core Facility) for help with quantitative mass spectrometry and the Whitehead Institute Metabolite Profiling Core Facility for measuring metabolite levels. Instrumentation resources from the Biophysical Instrumentation Facility for the Study of Complex Macromolecular Systems (NSF-0070319), the Structural Biology Core Facility, and the BioMicro Center in the Department of Biology at MIT are gratefully acknowledged. This work was supported by a fellowship from the Jane Coffin Childs Memorial Fund for Medical Research to B.W. and an NIH grant to M.T.L. (R01GM082899), who is also supported as an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
B.W., P.D., and B.L.P. designed and synthesized capture compounds. D.D. performed phylogenetic analyses. A.D.R. analyzed proteomics data. R.A.G. helped with X-ray structure determination. B.W. performed all other experiments. B.W. and M.T.L. designed experiments, analyzed data, prepared figures, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–9
Supplementary Note
Synthetic procedures
Supplementary Dataset
SILAC mass spectrometry results
Rights and permissions
About this article
Cite this article
Wang, B., Dai, P., Ding, D. et al. Affinity-based capture and identification of protein effectors of the growth regulator ppGpp. Nat Chem Biol 15, 141–150 (2019). https://doi.org/10.1038/s41589-018-0183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0183-4
This article is cited by
-
ppGpp accumulation reduces the expression of the global nitrogen homeostasis-modulating NtcA regulon by affecting 2-oxoglutarate levels
Communications Biology (2023)
-
Control of transcription elongation and DNA repair by alarmone ppGpp
Nature Structural & Molecular Biology (2023)
-
MESH1 knockdown triggers proliferation arrest through TAZ repression
Cell Death & Disease (2022)
-
Inhibition of SRP-dependent protein secretion by the bacterial alarmone (p)ppGpp
Nature Communications (2022)
-
Feedforward growth rate control mitigates gene activation burden
Nature Communications (2022)