Abstract
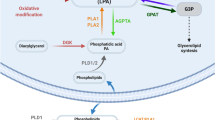
ABHD12 metabolizes bioactive lysophospholipids, including lysophosphatidylserine (lyso-PS). Deleterious mutations in human ABHD12 cause the neurological disease PHARC, and ABHD12–/– mice display PHARC-like phenotypes, including hearing loss, along with elevated brain lyso-PS and features of stimulated innate immune cell function. Here, we develop a selective and in vivo–active inhibitor of ABHD12 termed DO264 and show that this compound elevates lyso-PS in mouse brain and primary human macrophages. Unlike ABHD12–/– mice, adult mice treated with DO264 exhibited minimal perturbations in auditory function. On the other hand, both DO264-treated and ABHD12–/– mice displayed heightened immunological responses to lymphocytic choriomeningitis virus (LCMV) clone 13 infection that manifested as severe lung pathology with elevated proinflammatory chemokines. These results reveal similarities and differences in the phenotypic impact of pharmacological versus genetic blockade of ABHD12 and point to a key role for this enzyme in regulating immunostimulatory lipid pathways in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files) or are available from the corresponding author on reasonable request.
References
Rosen, H., Germana Sanna, M., Gonzalez-Cabrera, P. J. & Roberts, E. The organization of the sphingosine 1-phosphate signaling system. Curr. Top. Microbiol. Immunol. 378, 1–21 (2014).
Yung, Y. C., Stoddard, N. C. & Chun, J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J. Lipid Res. 55, 1192–1214 (2014).
Makide, K. et al. Novel lysophosphoplipid receptors: their structure and function. J. Lipid Res. 55, 1986–1995 (2014).
Kihara, Y., Mizuno, H. & Chun, J. Lysophospholipid receptors in drug discovery. Exp. Cell Res. 333, 171–177 (2015).
van der Kleij, D. et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002).
Gonzalez-Cabrera, P. J., Brown, S., Studer, S. M. & Rosen, H. S1P signaling: new therapies and opportunities. F1000Prime Rep. 6, 109 (2014).
Inoue, A. & Aoki, J. TGFalpha shedding assay is useful for identifying ligands for orphan GPCRs. Seikagaku 85, 1029–1033 (2013).
Kitamura, H. et al. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J. Biochem. 151, 511–518 (2012).
Barnes, M. J. et al. The lysophosphatidylserine receptor GPR174 constrains regulatory T cell development and function. J. Exp. Med. 212, 1011–1020 (2015).
Sugo, T. et al. Identification of a lysophosphatidylserine receptor on mast cells. Biochem. Biophys. Res. Commun. 341, 1078–1087 (2006).
Shinjo, Y. et al. Lysophosphatidylserine suppresses IL-2 production in CD4 T cells through LPS3/GPR174. Biochem. Biophys. Res. Commun. 494, 332–338 (2017).
Barnes, M. J. & Cyster, J. G. Lysophosphatidylserine suppression of T-cell activation via GPR174 requires Gαs proteins. Immunol. Cell Biol. 96, 439–445 (2018).
Kamat, S. S. et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat. Chem. Biol. 11, 164–171 (2015).
Sato, T. et al. Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J. Biol. Chem. 272, 2192–2198 (1997).
Blankman, J. L., Long, J. Z., Trauger, S. A., Siuzdak, G. & Cravatt, B. F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl Acad. Sci. USA 110, 1500–1505 (2013).
Fiskerstrand, T. et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: an inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 87, 410–417 (2010).
Fiskerstrand, T. et al. A novel Refsum-like disorder that maps to chromosome 20. Neurology 72, 20–27 (2009).
Parkkari, T. et al. Discovery of triterpenoids as reversible inhibitors of α/β-hydrolase domain containing 12 (ABHD12). PLoS One 9, e98286 (2014).
Hoover, H. S., Blankman, J. L., Niessen, S. & Cravatt, B. F. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg. Med. Chem. Lett. 18, 5838–5841 (2008).
Bachovchin, D.A. et al. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus library screening. Proc. Natl Acad. Sci. U.S.A. 107, 20941–20946 (2010).
Adibekian, A. et al. Click-generated triazole ureas as ultrapotent in vivo-active serine hydrolase inhibitors. Nat. Chem. Biol. 7, 469–478 (2011).
Savinainen, J. R., Navia-Paldanius, D. & Laitinen, J. T. A sensitive and versatile fluorescent activity assay for ABHD12. Methods Mol. Biol. 1412, 179–189 (2016).
van der Wel, T. et al. A natural substrate-based fluorescence assay for inhibitor screening on diacylglycerol lipase α. J. Lipid Res. 56, 927–935 (2015).
Leung, D., Hardouin, C., Boger, D. L. & Cravatt, B. F. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nat. Biotechnol. 21, 687–691 (2003).
Cognetta, A. B. III et al. Selective N-hydroxyhydantoin carbamate inhibitors of mammalian serine hydrolases. Chem. Biol. 22, 928–937 (2015).
Liu, Y. & Patricelli, M. P. & Cravatt, B. F. et al. Activity-based protein profiling: the serine hydrolases.Proc. Natl Acad. Sci. USA 96, 14694–14699 (1999).
Wu, C. et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 10, R130 (2009).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Viader, A. et al. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. eLife 5, e12345 (2016).
Gijón, M. A., Riekhof, W. R., Zarini, S., Murphy, R. C. & Voelker, D. R. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J. Biol. Chem. 283, 30235–30245 (2008).
Blankman, J. L., Simon, G. M. & Cravatt, B. F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14, 1347–1356 (2007).
Navia-Paldanius, D., Savinainen, J. R. & Laitinen, J. T. Biochemical and pharmacological characterization of human α/β-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12). J. Lipid Res. 53, 2413–2424 (2012).
Oldstone, M. B. Lessons learned and concepts formed from study of the pathogenesis of the two negative-strand viruses lymphocytic choriomeningitis and influenza. Proc. Natl Acad. Sci. USA 110, 4180–4183 (2013).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Brooks, D. G. et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12, 1301–1309 (2006).
Baccala, R. et al. Type I interferon is a therapeutic target for virus-induced lethal vascular damage. Proc. Natl Acad. Sci. USA 111, 8925–8930 (2014).
Frebel, H. et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209, 2485–2499 (2012).
Rabbani, B., Mahdieh, N., Hosomichi, K., Nakaoka, H. & Inoue, I. Next-generation sequencing: impact of exome sequencing in characterizing Mendelian disorders. J. Hum. Genet. 57, 621–632 (2012).
Sims, R. et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384 (2017).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Alhouayek, M., Masquelier, J. & Muccioli, G. G. Lysophosphatidylinositols, from cell membrane constituents to GPR55 ligands. Trends Pharmacol. Sci. 39, 586–604 (2018).
Olson, J. K. & Miller, S. D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 173, 3916–3924 (2004).
Neher, J. J. et al. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J. Immunol. 186, 4973–4983 (2011).
Lehnardt, S. et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 177, 583–592 (2006).
Hoffmann, O. et al. TLR2 mediates neuroinflammation and neuronal damage. J. Immunol. 178, 6476–6481 (2007).
Vambutas, A. & Pathak, S. AAO: autoimmune and autoinflammatory (disease) in otology: what is new in immune-mediated hearing loss. Laryngoscope Investig. Otolaryngol. 1, 110–115 (2016).
Wootla, B., Eriguchi, M. & Rodriguez, M. Is multiple sclerosis an autoimmune disease? Autoimmune Dis. 2012, 969657 (2012).
Suciu, R. M., Cognetta, A. B. III, Potter, Z. E. & Cravatt, B. F. Selective irreversible inhibitors of the Wnt-deacylating enzyme NOTUM developed by activity-based protein profiling. ACS Med. Chem. Lett. 9, 563–568 (2018).
Bachovchin, D. A. et al. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc. Natl Acad. Sci. USA 107, 20941–20946 (2010).
Alexander, J. P. & Cravatt, B. F. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem. Biol. 12, 1179–1187 (2005).
Martin, B. R., Wang, C., Adibekian, A., Tully, S. E. & Cravatt, B. F. Global profiling of dynamic protein palmitoylation. Nat. Methods 9, 84–89 (2011).
Hulce, J. J., Cognetta, A. B., Niphakis, M. J., Tully, S. E. & Cravatt, B. F. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods 10, 259–264 (2013).
Ogasawara, D. et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc. Natl Acad. Sci. USA 113, 26–33 (2016).
Borrow, P., Evans, C. F. & Oldstone, M. B. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69, 1059–1070 (1995).
Ahmed, R., Salmi, A., Butler, L. D., Chiller, J. M. & Oldstone, M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Acknowledgements
This work was supported by the NIH (DA033760, NS092980, AI123210, AI117175) and the Skaggs Institute for Chemical Biology. J.B. is supported by a fellowship from Celgene. We thank J. Olucha for recombinant expression of mABHD12 in HEK293F cells; J. Chen (Automated Synthesis Facility at Scripps Research) for measuring enantiopurity of (S)-DO271; C.E. Moore and M. Gembicky (UCSD) for X-ray crystallographic analysis of DO253; and J. Wang and C. Chen (Pharmaron) for the measurement of tissue drug concentration.
Author information
Authors and Affiliations
Contributions
D.O., T.-A.I., V.F.V., J.R.T., and B.F.C. conceived the project and designed the experiments. T.-A.I. performed the HTS study. S.B. assisted with HTS assay development, and H.R. provided the small molecule library and screening instruments. D.O. synthesized and chemically characterized the compounds. J.J.H. synthesized JJH350. D.O., T.-A.I., A.R., O.U., and H.J. performed the biochemical and cellular experiments. J.B. performed the experiments with primary human macrophages. D.O. and T.-A.I. performed the in vivo compound dosing and lipidomic studies. A.R. performed the auditory tests. V.F.V. and J.R.T. performed the immunological experiments. T.-A.I., D.O., J.R.T. and B.F.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
O.U. and A.R. are employees of and B.F.C. is a founder and advisor to Abide Therapeutics, a biotechnology company interested in developing serine hydrolase inhibitors and therapeutics.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–2, Supplementary Figures 1–29
Supplementary Note
Synthetic Procedures
Supplementary Table 3
Complete mass spectrometry–based ABPP data
Supplementary Table 4
Complete targeted lipidomics data
Rights and permissions
About this article
Cite this article
Ogasawara, D., Ichu, TA., Vartabedian, V.F. et al. Selective blockade of the lyso-PS lipase ABHD12 stimulates immune responses in vivo. Nat Chem Biol 14, 1099–1108 (2018). https://doi.org/10.1038/s41589-018-0155-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0155-8
This article is cited by
-
Genetic insights into PHARC syndrome: identification of a novel frameshift mutation in ABHD12
BMC Medical Genomics (2023)
-
Specific binding of GPR174 by endogenous lysophosphatidylserine leads to high constitutive Gs signaling
Nature Communications (2023)
-
Increase in Cellular Lysophosphatidylserine Content Exacerbates Inflammatory Responses in LPS-Activated Microglia
Neurochemical Research (2022)
-
Genetic architecture and genomic selection of fatty acid composition predicted by Raman spectroscopy in rainbow trout
BMC Genomics (2021)
-
G-protein-coupled receptor P2Y10 facilitates chemokine-induced CD4 T cell migration through autocrine/paracrine mediators
Nature Communications (2021)