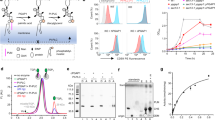
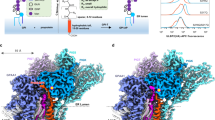
Abstract
Polyprenol phosphate phosphoglycosyl transferases (PGTs) catalyze the first membrane-committed step in assembly of essential glycoconjugates. Currently there is no structure–function information to describe how monotopic PGTs coordinate the reaction between membrane-embedded and soluble substrates. We describe the structure and mode of membrane association of PglC, a PGT from Campylobacter concisus. The structure reveals a unique architecture, provides mechanistic insight and identifies ligand-binding determinants for PglC and the monotopic PGT superfamily.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lukose, V., Walvoort, M. T. C. & Imperiali, B. Glycobiology 27, 820–833 (2017).
Price, N. P. & Momany, F. A. Glycobiology 15, 29R–42R (2005).
Chung, B. C. et al. Science 341, 1012–1016 (2013).
Lukose, V. et al. Biochemistry 54, 7326–7334 (2015).
Tytgat, H. L. & Lebeer, S. Microbiol. Mol. Biol. Rev. 78, 372–417 (2014).
Bugg, T. D., Rodolis, M. T., Mihalyi, A. & Jamshidi, S. Bioorg. Med. Chem. 24, 6340–6347 (2016).
Das, D., Kuzmic, P. & Imperiali, B. Proc. Natl Acad. Sci. USA 114, 7019–7024 (2017).
Al-Dabbagh, B. et al. Biochimie. 127, 249–257 (2016).
Hartley, M. D., Schneggenburger, P. E. & Imperiali, B. Proc. Natl Acad. Sci. USA 110, 20863–20870 (2013).
Holm, L. & Rosenstrom, P. Nucleic Acids Res. 38, W545–W549 (2010).
Aoki, S., Thomas, A., Decaffmeyer, M., Brasseur, R. & Epand, R. M. J. Biol. Chem. 285, 33371–33380 (2010).
Heijne, G. EMBO J. 5, 3021–3027 (1986).
Furlong, S. E., Ford, A., Albarnez-Rodriguez, L. & Valvano, M. A. Sci. Rep. 5, 9178 (2015).
Patel, K. B., Ciepichal, E., Swiezewska, E. & Valvano, M. A. Glycobiology 22, 116–122 (2012).
Nasie, I., Steiner-Mordoch, S. & Schuldiner, S. Methods Mol. Biol. 1033, 121–130 (2013).
Eisenberg, D., Schwarz, E., Komaromy, M. & Wall, R. J. Mol. Biol. 179, 125–142 (1984).
Zidovetzki, R., Rost, B., Armstrong, D. L. & Pecht, I. Biophys. Chem. 100, 555–575 (2003).
Jones, S., Daley, D. T., Luscombe, N. M., Berman, H. M. & Thornton, J. M. Nucleic Acids Res. 29, 943–954 (2001).
Lloyd, A. J., Brandish, P. E., Gilbey, A. M. & Bugg, T. D. H. J. Bacteriol. 186, 1747–1757 (2004).
Amer, A. O. & Valvano, M. A. Microbiology 148, 571–582 (2002).
Pace, C. N. & Scholtz, J. M. Biophys. J. 75, 422–427 (1998).
Allen, K. N. & Dunaway-Mariano, D. Curr. Opin. Struct. Biol. 41, 172–179 (2016).
Ardiccioni, C. et al. Nat. Commun. 7, 10175 (2016).
Studier, F. W. Protein Expr. Purif. 41, 207–234 (2005).
Weeks, S. D., Drinker, M. & Loll, P. J. Protein Expr. Purif. 53, 40–50 (2007).
Koszelak-Rosenblum, M. et al. Protein Sci. 18, 1828–1839 (2009).
Adams, P. D. et al. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Schneider, T. R. & Sheldrick, G. M. Acta Crystallogr. D Biol. Crystallogr 58, 1772–1779 (2002).
Terwilliger, T. C. & Acta Crystallogr., D. Biol. Crystallogr. 56, 965–972 (2000).
Wang, S., Sun, S., Li, Z., Zhang, R. & Xu, J. PLoS Comput. Biol. 13, e1005324 (2017).
Ovchinnikov, S. et al. Science 355, 294–298 (2017).
McCoy, A. J. et al. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Acta. Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Acta. Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Acknowledgements
We thank K. Rajashankar for assistance with phasing and the staff at NECAT (APS) for facilitating X-ray data collection. Financial support for this work was provided by the National Institutes of Health: R01-GM039334 to B.I., the Predoctoral Training Program in the Biological Sciences (T32-GM007287) to S.E. and the Biomolecular Pharmacology Program Grant (T32-GM008541) to L.C.R. This work is also based upon research conducted at the Northeastern Collaborative Access Team beamlines 24-ID-E and 24-ID-C, which is funded by the National Institute of General Medical Sciences from the National Institutes of Health (P41 GM103403).
Author information
Authors and Affiliations
Contributions
L.C.R. crystallized, collected data, determined the structure, refined and analyzed the model of PglC and performed phosphate release kinetics; D.D. optimized expression, designed and made constructs, expressed and purified PglC, carried out lipid analysis and analyzed the structure. S.E. designed and performed SCAM analyses. V.L. designed and purified original constructs for crystallization and A.J.L. obtained the original crystallization conditions. L.C.R., D.D. and S.E. wrote the manuscript. B.I. and K.N.A. conceived the project, designed experiments, assisted with data analysis and interpretation, and critically edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1–5
Dataset 1
Supplementary Dataset 1
Rights and permissions
About this article
Cite this article
Ray, L.C., Das, D., Entova, S. et al. Membrane association of monotopic phosphoglycosyl transferase underpins function. Nat Chem Biol 14, 538–541 (2018). https://doi.org/10.1038/s41589-018-0054-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0054-z
This article is cited by
-
Structural insights into phosphatidylethanolamine formation in bacterial membrane biogenesis
Scientific Reports (2021)
-
Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus
Nature Communications (2019)