Abstract
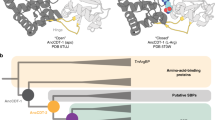
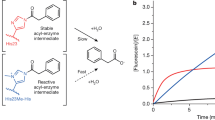
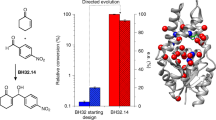
The emergence of enzymes through the neofunctionalization of noncatalytic proteins is ultimately responsible for the extraordinary range of biological catalysts observed in nature. Although the evolution of some enzymes from binding proteins can be inferred by homology, we have a limited understanding of the nature of the biochemical and biophysical adaptations along these evolutionary trajectories and the sequence in which they occurred. Here we reconstructed and characterized evolutionary intermediate states linking an ancestral solute-binding protein to the extant enzyme cyclohexadienyl dehydratase. We show how the intrinsic reactivity of a desolvated general acid was harnessed by a series of mutations radiating from the active site, which optimized enzyme–substrate complementarity and transition-state stabilization and minimized sampling of noncatalytic conformations. Our work reveals the molecular evolutionary processes that underlie the emergence of enzymes de novo, which are notably mirrored by recent examples of computational enzyme design and directed evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baier, F., Copp, J. N. & Tokuriki, N. Evolution of enzyme superfamilies: comprehensive exploration of sequence-function relationships. Biochemistry 55, 6375–6388 (2016).
Khersonsky, O. & Tawfik, D. S. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu. Rev. Biochem. 79, 471–505 (2010).
Furnham, N., Dawson, N. L., Rahman, S. A., Thornton, J. M. & Orengo, C. A. Large-scale analysis exploring evolution of catalytic machineries and mechanisms in enzyme superfamilies. J. Mol. Biol. 428 2 Pt A, 253–267 (2016).
Harms, M. J. & Thornton, J. W. Evolutionary biochemistry: revealing the historical and physical causes of protein properties. Nat. Rev. Genet. 14, 559–571 (2013).
Tam, R. & Saier, M. H. Jr. A bacterial periplasmic receptor homologue with catalytic activity: cyclohexadienyl dehydratase of Pseudomonas aeruginosa is homologous to receptors specific for polar amino acids. Res. Microbiol. 144, 165–169 (1993).
Ngaki, M. N. et al. Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 485, 530–533 (2012).
Ortmayer, M. et al. An oxidative N-demethylase reveals PAS transition from ubiquitous sensor to enzyme. Nature 539, 593–597 (2016).
Zhao, G. S., Xia, T. H., Fischer, R. S. & Jensen, R. A. Cyclohexadienyl dehydratase from Pseudomonas aeruginosa. Molecular cloning of the gene and characterization of the gene product. J. Biol. Chem. 267, 2487–2493 (1992).
Berntsson, R. P.-A., Smits, S. H. J., Schmitt, L., Slotboom, D.-J. & Poolman, B. A structural classification of substrate-binding proteins. FEBS Lett. 584, 2606–2617 (2010).
Hochberg, G. K. A. & Thornton, J. W. Reconstructing ancient proteins to understand the causes of structure and function. Annu. Rev. Biophys. 46, 247–269 (2017).
Vetting, M. W. et al. Experimental strategies for functional annotation and metabolism discovery: targeted screening of solute binding proteins and unbiased panning of metabolomes. Biochemistry 54, 909–931 (2015).
Gouridis, G. et al. Conformational dynamics in substrate-binding domains influences transport in the ABC importer GlnPQ. Nat. Struct. Mol. Biol. 22, 57–64 (2015).
Marvin, J. S. & Hellinga, H. W. Manipulation of ligand binding affinity by exploitation of conformational coupling. Nat. Struct. Mol. Biol. 8, 795–798 (2001).
Campbell, E. et al. The role of protein dynamics in the evolution of new enzyme function. Nat. Chem. Biol. 12, 944–950 (2016).
Bar-Even, A., Milo, R., Noor, E. & Tawfik, D. S. The moderately efficient enzyme: futile encounters and enzyme floppiness. Biochemistry 54, 4969–4977 (2015).
Bermejo, G. A., Strub, M.-P., Ho, C. & Tjandra, N. Ligand-free open-closed transitions of periplasmic binding proteins: the case of glutamine-binding protein. Biochemistry 49, 1893–1902 (2010).
Silva, D.-A., Domínguez-Ramírez, L., Rojo-Domínguez, A. & Sosa-Peinado, A. Conformational dynamics of l-lysine, l-arginine, l-ornithine binding protein reveals ligand-dependent plasticity. Proteins 79, 2097–2108 (2011).
Chu, B. C. H., Chan, D. I., DeWolf, T., Periole, X. & Vogel, H. J. Molecular dynamics simulations reveal that apo-HisJ can sample a closed conformation. Proteins 82, 386–398 (2014).
Salverda, M. L. M. et al. Initial mutations direct alternative pathways of protein evolution. PLoS Genet. 7, e1001321 (2011).
Kaltenbach, M., Jackson, C. J., Campbell, E. C., Hollfelder, F. & Tokuriki, N. Reverse evolution leads to genotypic incompatibility despite functional and active site convergence. eLife 4, e06492 (2015).
Sugrue, E., Carr, P. D., Scott, C. & Jackson, C. J. Active site desolvation and thermostability tradeoffs in the evolution of catalytically diverse triazine hydrolases. Biochemistry 55, 6304–6313 (2016).
Moroz, Y. S. et al. New tricks for old proteins: single mutations in a non-enzymatic protein give rise to various enzymatic activities. J. Am. Chem. Soc. 137, 14905–14911 (2015).
Tokuriki, N. et al. Diminishing returns and tradeoffs constrain the laboratory optimization of an enzyme. Nat. Commun. 3, 1257 (2012).
Anderson, D. P. et al. Evolution of an ancient protein function involved in organized multicellularity in animals. eLife 5, e10147 (2016).
Huang, P.-S., Boyken, S. E. & Baker, D. The coming of age of de novo protein design. Nature 537, 320–327 (2016).
Burton, A. J., Thomson, A. R., Dawson, W. M., Brady, R. L. & Woolfson, D. N. Installing hydrolytic activity into a completely de novo protein framework. Nat. Chem. 8, 837–844 (2016).
Röthlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature 453, 190–195 (2008).
Mak, W. S. & Siegel, J. B. Computational enzyme design: transitioning from catalytic proteins to enzymes. Curr. Opin. Struct. Biol. 27, 87–94 (2014).
Korendovych, I. V. & DeGrado, W. F. Catalytic efficiency of designed catalytic proteins. Curr. Opin. Struct. Biol. 27, 113–121 (2014).
Blomberg, R. et al. Precision is essential for efficient catalysis in an evolved Kemp eliminase. Nature 503, 418–421 (2013).
Khersonsky, O. et al. Bridging the gaps in design methodologies by evolutionary optimization of the stability and proficiency of designed Kemp eliminase KE59. Proc. Natl. Acad. Sci. USA 109, 10358–10363 (2012).
Engler, C., Kandzia, R. & Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS One 3, e3647 (2008).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Clifton, B. E. & Jackson, C. J. Ancestral protein reconstruction yields insights into adaptive evolution of binding specificity in solute-binding proteins. Cell Chem. Biol. 23, 236–245 (2016).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Abascal, F., Zardoya, R. & Posada, D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 2104–2105 (2005).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
McKellar, J. L., Minnell, J. J. & Gerth, M. L. A high-throughput screen for ligand binding reveals the specificities of three amino acid chemoreceptors from Pseudomonas syringae pv. actinidiae. Mol. Microbiol. 96, 694–707 (2015).
Gibson, F. Chorismic acid: purification and some chemical and physical studies. Biochem. J. 90, 256–261 (1964).
Gibson, M. I. & Gibson, F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem. J. 90, 248–256 (1964).
McPhillips, T. M. et al. Blu-Ice and the Distributed Control System: software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron Radiat. 9, 401–406 (2002).
Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011).
Kabsch, W. XDS. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Zhao, H. & Zha, W. In vitro ‘sexual’ evolution through the PCR-based staggered extension process (StEP). Nat. Protoc. 1, 1865–1871 (2006).
Herman, A. & Tawfik, D. S. Incorporating Synthetic Oligonucleotides via Gene Reassembly (ISOR): a versatile tool for generating targeted libraries. Protein Eng. Des. Sel. 20, 219–226 (2007).
Rockah-Shmuel, L., Tawfik, D. S. & Goldsmith, M. in Directed Evolution Library Creation: Methods and Protocols (eds. Gillam, E. M. J., Copp, J. N. & Ackerley, D. F.) Vol. 1179, 129–137 (Springer-Verlag, 2014).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013).
Oostenbrink, C., Villa, A., Mark, A. E. & van Gunsteren, W. F. A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 25, 1656–1676 (2004).
Bowers, K. et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. Proc. ACM/IEEE SC Conf. Supercomput. (SC06) (ACM, Tampa, Florida, 2006).
Harder, E. et al. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 12, 281–296 (2016).
Grant, B. J., Rodrigues, A. P. C., ElSawy, K. M., McCammon, J. A. & Caves, L. S. D. Bio3d: an R package for the comparative analysis of protein structures. Bioinformatics 22, 2695–2696 (2006).
Hayward, S. & Berendsen, H. J. Systematic analysis of domain motions in proteins from conformational change: new results on citrate synthase and T4 lysozyme. Proteins 30, 144–154 (1998).
Olsson, M. H. M., Søndergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa calculations. J. Chem. Theory Comput. 7, 525–537 (2011).
Acknowledgements
B.E.C. and J.A.K. were supported by Australian Postgraduate Awards. B.E.C. was also supported by a Rod Rickards PhD scholarship and an Alan Sargeson scholarship. This research was undertaken with the assistance of resources, services, and staff from the Australian National Computational Infrastructure (NCI), the Australian Synchrotron, and the CSIRO Collaborative Crystallisation Centre, and funding from the Australian Research Council Discovery Project scheme (C.J.J.). We thank A. Saeed, P. Yates, L. Tan and S. Warring for additional technical contributions. We thank H. Janovjak (IST Austria) for gifting us the pDOTS7 plasmid.
Author information
Authors and Affiliations
Contributions
B.E.C. and C.J.J. conceived the study; B.E.C. and J.A.K. performed computational analysis; J.A.K., B.E.C., and M.L.G. performed experimental characterization of proteins; B.E.C., J.A.K., P.D.C., and C.J.J. solved the crystal structures; N. T. and C.J.J. supervised students; B.E.C., J.A.K., and C.J.J. wrote the paper. All authors contributed to experimental design, editing of the paper, and interpretation of results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1–13, Supplementary Figures 1–13
Supplementary Dataset 1
Ligand screening of Pu1068 and AncCDT-2 by differential scanning fluorimetry
Rights and permissions
About this article
Cite this article
Clifton, B.E., Kaczmarski, J.A., Carr, P.D. et al. Evolution of cyclohexadienyl dehydratase from an ancestral solute-binding protein. Nat Chem Biol 14, 542–547 (2018). https://doi.org/10.1038/s41589-018-0043-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0043-2
This article is cited by
-
Genome-wide identification of rubber tree pathogenesis-related 10 (PR-10) proteins with biological relevance to plant defense
Scientific Reports (2024)
-
Extant Sequence Reconstruction: The Accuracy of Ancestral Sequence Reconstructions Evaluated by Extant Sequence Cross-Validation
Journal of Molecular Evolution (2024)
-
Evolution of enzyme functionality in the flavin-containing monooxygenases
Nature Communications (2023)
-
Loop dynamics and the evolution of enzyme activity
Nature Reviews Chemistry (2023)
-
Computational remodeling of an enzyme conformational landscape for altered substrate selectivity
Nature Communications (2023)