Abstract
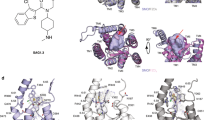
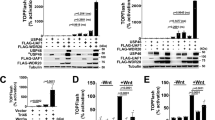
Regeneration of the adult intestinal epithelium is mediated by a pool of cycling stem cells, which are located at the base of the crypt, that express leucine-rich-repeat-containing G-protein-coupled receptor 5 (LGR5). The Frizzled (FZD) 7 receptor (FZD7) is enriched in LGR5+ intestinal stem cells and plays a critical role in their self-renewal. Yet, drug discovery approaches and structural bases for targeting specific FZD isoforms remain poorly defined. FZD proteins interact with Wnt signaling proteins via, in part, a lipid-binding groove on the extracellular cysteine-rich domain (CRD) of the FZD receptor. Here we report the identification of a potent peptide that selectively binds to the FZD7 CRD at a previously uncharacterized site and alters the conformation of the CRD and the architecture of its lipid-binding groove. Treatment with the FZD7-binding peptide impaired Wnt signaling in cultured cells and stem cell function in intestinal organoids. Together, our data illustrate that targeting the lipid-binding groove holds promise as an approach for achieving isoform-selective FZD receptor inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
04 May 2018
The version of this article originally published contained older versions of the Life Sciences Reporting Summary and the Supplementary Text and Figures. The error has been corrected in the HTML and PDF versions of the article.
References
Mariadason, J. M. et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt–villus axis. Gastroenterology 128, 1081–1088 (2005).
Gregorieff, A. et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638 (2005).
Flanagan, D. J. et al. Frizzled 7 functions as a Wnt receptor in intestinal epithelial LGR5+ stem cells. Stem Cell Rep. 4, 759–767 (2015).
Fernandez, A. et al. The Wnt receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 111, 1409–1414 (2014).
Vincan, E. & Barker, N. The upstream components of the Wnt signaling pathway in the dynamic EMT and MET associated with colorectal cancer progression. Clin. Exp. Metastasis 25, 657–663 (2008).
Phesse, T., Flanagan, D. & Vincan, E. Frizzled 7: a promising Achilles’ heel for targeting the Wnt receptor complex to treat cancer. Cancers 8, E50 (2016).
Tiwary, S. & Xu, L. Frizzled 7 is required for tumor initiation and metastatic growth of melanoma cells. PLoS One 11, e0147638 (2016).
Anastas, J. N. et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J. Clin. Invest. 124, 2877–2890 (2014).
Janda, C. Y., Waghray, D., Levin, A. M., Thomas, C. & Garcia, K. C. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012).
Nile, A. H. & Hannoush, R. N. Fatty acylation of Wnt proteins. Nat. Chem. Biol. 12, 60–69 (2016).
Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 4, 1–13 (2012).
Giannakis, M. et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 46, 1264–1266 (2014).
Jiang, X., Charlat, O., Zamponi, R., Yang, Y. & Cong, F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3 and RNF43 E3 ubiquitin ligases. Mol. Cell 58, 522–533 (2015).
Koo, B. K. et al. Tumor suppressor RNF43 is a stem cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012).
Jiang, X. et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. USA 110, 12649–12654 (2013).
Koo, B.-K., van Es, J. H., van den Born, M. & Clevers, H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutated neoplasia. Proc. Natl. Acad. Sci. USA 112, 7548–7550 (2015).
Dijksterhuis, J. P. et al. Systematic mapping of Wnt–FZD protein interactions reveals functional selectivity by distinct Wnt–FZD pairs. J. Biol. Chem. 290, 6789–6798 (2015).
Lee, H. J. et al. Structure-based discovery of novel small-molecule Wnt signaling inhibitors by targeting the cysteine-rich domain of Frizzled. J. Biol. Chem. 290, 30596–30606 (2015).
Gurney, A. et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 109, 11717–11722 (2012).
Hannoush, R. N. Kinetics of Wnt-driven β-catenin stabilization revealed by quantitative and temporal imaging. PLoS One 3, e3498 (2008).
Meijer, L. et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255–1266 (2003).
Steinhart, Z. et al. Genome-wide CRISPR screens reveal a Wnt–FZD5 signaling circuit as a druggable vulnerability of RNF43-mutated pancreatic tumors. Nat. Med. 23, 60–68 (2017).
Nile, A. H., Mukund, S., Stanger, K., Wang, W. & Hannoush, R. N. Unsaturated fatty acyl recognition by Frizzled receptors mediates dimerization upon Wnt ligand binding. Proc. Natl. Acad. Sci. USA 114, 4147–4152 (2017).
Dann, C. E. et al. Insights into Wnt binding and signaling from the structures of two Frizzled cysteine-rich domains. Nature 412, 86–90 (2001).
Shen, G. et al. Structural basis of the norrin–Frizzled 4 interaction. Cell Res. 25, 1078–1081 (2015).
Bourhis, E. et al. Reconstitution of a Frizzled 8–WNT3A–LRP6 signaling complex reveals multiple Wnt and DKK1 binding sites on LRP6. J. Biol. Chem. 285, 9172–9179 (2010).
Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254 (2016).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Grabinger, T. et al. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 5, e1228 (2014).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 (2013).
Janda, C. Y. et al. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signaling. Nature 545, 234–237 (2017).
Voloshanenko, O., Gmach, P., Winter, J., Kranz, D. & Boutros, M. Mapping of Wnt–Frizzled interactions by multiplex CRISPR targeting of receptor gene families. FASEB J. 31, 4832–4844 (2017).
Najdi, R. et al. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation 84, 203–213 (2012).
Tian, H. et al. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep. 11, 33–42 (2015).
Stanger, K. et al. Allosteric peptides bind a caspase zymogen and mediate caspase tetramerization. Nat. Chem. Biol. 8, 655–660 (2012).
Tonikian, R., Zhang, Y., Boone, C. & Sidhu, S. S. Identifying specificity profiles for peptide recognition modules from phage-displayed peptide libraries. Nat. Protoc. 2, 1368–1386 (2007).
Zhang, Y. et al. Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nat. Chem. Biol. 5, 217–219 (2009).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 (2010).
Blessing, R. H. An empirical correction for absorption anisotropy. Acta Crystallogr. A 51, 33–38 (1995).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005).
Wüthrich, K. NMR of Proteins and Nucleic Acids (Wiley Interscience, New York, 1986).
Güntert, P. & Buchner, L. Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 62, 453–471 (2015).
Güntert, P., Mumenthaler, C. & Wüthrich, K. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273, 283–298 (1997).
Brünger, A. T. et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Brunger, A. T. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2, 2728–2733 (2007).
Sato, T. et al. Single LGR5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
de Sousa e Melo, F. et al. A distinct role for LGR5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Wu, T. D. & Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26, 873–881 (2010).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Srinivasan, K. et al. Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat. Commun. 7, 11295 (2016).
Yaari, G., Bolen, C. R., Thakar, J. & Kleinstein, S. H. Quantitative set analysis for gene expression: a method to quantify gene set differential expression including gene–gene correlations. Nucleic Acids Res. 41, e170 (2013).
Kim, T.-H. et al. Single-cell transcript profiles reveal multilineage priming in early progenitors derived from LGR5+ intestinal stem cells. Cell Rep. 16, 2053–2060 (2016).
Acknowledgements
We thank R. Ferrao for assistance with collecting the crystallographic dataset, S. Gierke for help with microscopy, A. Estevez, C. Ciferri, K. Mortara and the Baculovirus expression group for assistance with protein expression, R. Ferrao and D. Whalen for helpful discussions, and the Genentech FACS core for technical assistance. Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera was developed by the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco (supported by US National Institutes of Health (NIH)-NIGMS grant no. P41-GM103311).
Author information
Authors and Affiliations
Contributions
A.H.N., F.d.S.e.M., S.M., R.P., S.H., L.Z., Y.Z., Y.F., E.B.G., Z.M., S.A., Y.F., C.K., W.J.F., W.W., F.J.d.S. and R.N.H. designed research; A.H.N. performed protein purification and characterization, and biochemical and cellular assays; F.d.S.e.M. performed organoid assays; S.M. performed crystallography experiments; S.H. performed SPR experiments; L.Z. performed phage panning; Y.F. designed expression constructs; E.B.G. and R.N.H. performed cellular assays; L.G.K. performed confocal microscopy; A.H.N. and W.W. solved the X-ray crystal structure; R.P. analyzed the RNA-seq data; A.H.N. and R.N.H. wrote the manuscript, with input from the other authors; and R.N.H. conceived and directed the study.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of Genentech, a member of the Roche group. A patent (PCT/US2017/050841; A.H.N., Y.Z., L.Z., and R.N.H.) covering this work is pending.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–4 and Supplementary Figures 1–25
Rights and permissions
About this article
Cite this article
Nile, A.H., de Sousa e Melo, F., Mukund, S. et al. A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat Chem Biol 14, 582–590 (2018). https://doi.org/10.1038/s41589-018-0035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0035-2
This article is cited by
-
Senescent cells perturb intestinal stem cell differentiation through Ptk7 induced noncanonical Wnt and YAP signaling
Nature Communications (2023)
-
L-glutamate requires β-catenin signalling through Frizzled7 to stimulate porcine intestinal stem cell expansion
Cellular and Molecular Life Sciences (2022)
-
The Wnt signaling pathway in tumorigenesis, pharmacological targets, and drug development for cancer therapy
Biomarker Research (2021)
-
Organoid-based modeling of intestinal development, regeneration, and repair
Cell Death & Differentiation (2021)
-
Investigation of Atrial Natriuretic Peptide as A Competitive Inhibitory Candidate Against Wnt/β-Catenin Signalling: A Molecular Dynamics Approach
International Journal of Peptide Research and Therapeutics (2021)