Abstract
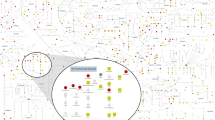
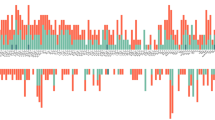
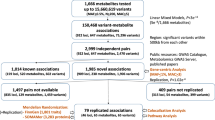
Metabolic processes can influence disease risk and provide therapeutic targets. By conducting genome-wide association studies of 1,091 blood metabolites and 309 metabolite ratios, we identified associations with 690 metabolites at 248 loci and associations with 143 metabolite ratios at 69 loci. Integrating metabolite-gene and gene expression information identified 94 effector genes for 109 metabolites and 48 metabolite ratios. Using Mendelian randomization (MR), we identified 22 metabolites and 20 metabolite ratios having estimated causal effect on 12 traits and diseases, including orotate for estimated bone mineral density, α-hydroxyisovalerate for body mass index and ergothioneine for inflammatory bowel disease and asthma. We further measured the orotate level in a separate cohort and demonstrated that, consistent with MR, orotate levels were positively associated with incident hip fractures. This study provides a valuable resource describing the genetic architecture of metabolites and delivers insights into their roles in common diseases, thereby offering opportunities for therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The GWAS summary statistics were deposited to GWAS catalog (https://www.ebi.ac.uk/gwas/). Accession numbers for European GWASs: GCST90199621-90201020; accession numbers for non-European GWASs: GCST90201021-90204063. Individual-level data are available from the Canadian Longitudinal Study on Aging (www.clsa-elcv.ca) for researchers who meet the criteria for access to de-identified CLSA data. HMDB database can be found in http://www.hmdb.ca/system/downloads/current/serum_metabolites.zip. KEGG pathway database can be found in http://hgdownload.cse.ucsc.edu/goldenpath/hg19/database/keggPathway.txt.gz. PubChem database can be found in https://pubchem.ncbi.nlm.nih.gov/. GTEx V8 release data can be found in https://www.gtexportal.org/home/datasets. UniProt database can be found in https://www.uniprot.org/uniprot/. GENCODE resource can be found in https://www.gencodegenes.org/. OMIM database can be found in https://www.omim.org/downloads. IMPC database can be found in https://www.mousephenotype.org/data/release. DrugBank database can be found in https://go.drugbank.com.
Code availability
The GWAS was performed using GCTA-fastGWA (v1.93.2 beta). Multi-SNP-based conditional and joint association analysis was performed using GCTA-COJO (v1.93.2 beta). bedtools version v2.29.2 was used. KING package (v2.2.5) was used to remove individuals with first- and second-degree relatives. Snappy (available through Zenodo (https://doi.org/10.5281/zenodo.7328428; ref. 77)) was used to identify LD-proxy SNPs. PLINK 1.9 was used to identify LD-independent SNPs from the trait and disease GWAS. All other data analyses were performed using R (version 4.0.5). R packages including dplyr (1.0.7), data.table (1.14.2), tidyverse (1.2.0), stringr (1.4.1), LDlinkR (1.1.2), TwoSampleMR (0.5.6), coloc (5.1.0), circlize (0.4.13), ComplexHeatmap (2.13.1), RcolorBrewer (1.1–3), ggpubr (0.4.0) and ggplot2 (3.3.5) were used for analysis and plotting. Other analyses and plotting scripts were made available through the GitHub repository (https://github.com/richardslab/metabolomics_GWAS_CLSA) and also through Zenodo (https://doi.org/10.5281/zenodo.7331471) (ref. 79).
References
Bar, N. et al. A reference map of potential determinants for the human serum metabolome. Nature 588, 135–140 (2020).
Lee, W.-J. & Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 10, 416–424 (2014).
Pietzner, M. et al. Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat. Med. 27, 471–479 (2021).
Wishart, D. S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 15, 473–484 (2016).
Long, T. et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet. 49, 568–578 (2017).
Hagenbeek, F. A. et al. Heritability estimates for 361 blood metabolites across 40 genome-wide association studies. Nat. Commun. 11, 39 (2020).
Smith, G. D. Mendelian randomization: prospects, potentials, and limitations. Int. J. Epidemiol. 33, 30–42 (2004).
Lotta, L. A. et al. A cross-platform approach identifies genetic regulators of human metabolism and health. Nat. Genet. 53, 54–64 (2021).
Feofanova, E. V. et al. A genome-wide association study discovers 46 loci of the human metabolome in the Hispanic community health study/study of Latinos. Am. J. Hum. Genet. 107, 849–863 (2020).
Shin, S.-Y. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550 (2014).
Yin, X. et al. Genome-wide association studies of metabolites in Finnish men identify disease-relevant loci. Nat. Commun. 13, 1644 (2022).
Kettunen, J. et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 7, 11122 (2016).
Draisma, H. H. M. et al. Genome-wide association study identifies novel genetic variants contributing to variation in blood metabolite levels. Nat. Commun. 6, 7208 (2015).
Kastenmüller, G., Raffler, J., Gieger, C. & Suhre, K. Genetics of human metabolism: an update. Hum. Mol. Genet. 24, R93–R101 (2015).
Yazdani, A., Yazdani, A., Liu, X. & Boerwinkle, E. Identification of rare variants in metabolites of the carnitine pathway by whole genome sequencing analysis. Genet. Epidemiol. 40, 486–491 (2016).
Yu, B. et al. Loss-of-function variants influence the human serum metabolome. Sci. Adv. 2, e1600800 (2016).
Yousri, N. A. et al. Whole-exome sequencing identifies common and rare variant metabolic QTLs in a Middle Eastern population. Nat. Commun. 9, 333 (2018).
Rhee, E. P. et al. An exome array study of the plasma metabolome. Nat. Commun. 7, 12360 (2016).
Illig, T. et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 42, 137–141 (2010).
Gieger, C. et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. 4, e1000282 (2008).
Hysi, P. G. et al. Metabolome genome-wide association study identifies 74 novel genomic regions influencing plasma metabolites levels. Metabolites 12, 61 (2022).
Bomba, L. et al. Whole-exome sequencing identifies rare genetic variants associated with human plasma metabolites. Am. J. Hum. Genet. https://doi.org/10.1016/j.ajhg.2022.04.009 (2022).
Raina, P. et al. Cohort profile: the Canadian Longitudinal Study on Aging (CLSA). Int. J. Epidemiol. 48, 1752–1753 (2019).
Kamat, M. A. et al. PhenoScanner V2: an expanded tool for searching human genotype–phenotype associations. Bioinformatics 35, 4851–4853 (2019).
Gallois, A. et al. A comprehensive study of metabolite genetics reveals strong pleiotropy and heterogeneity across time and context. Nat. Commun. 10, 4788 (2019).
Wishart, D. S. et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608–D617 (2018).
Petersen, A.-K. et al. On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinform. 13, 120 (2012).
Suhre, K. et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 477, 54–60 (2011).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kim, S. et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 49, D1388–D1395 (2021).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Dickinson, M. E. et al. High-throughput discovery of novel developmental phenotypes. Nature 537, 508–514 (2016).
Amberger, J. S., Bocchini, C. A., Scott, A. F. & Hamosh, A. OMIM.org: leveraging knowledge across phenotype–gene relationships. Nucleic Acids Res. 47, D1038–D1043 (2019).
Morris, J. A. et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 51, 258–266 (2019).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51, 404–413 (2019).
Tachmazidou, I. et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 51, 230–236 (2019).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174 (2019).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Malik, R. et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 50, 524–537 (2018).
van der Harst, P. & Verweij, N. Identification of 64 Novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ. Res. 122, 433–443 (2018).
Han, Y. et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat. Commun. 11, 1776 (2020).
Chiou, J. et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 594, 398–402 (2021).
de Lange, K. M. et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 49, 256–261 (2017).
International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188 (2019).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Zahoor, I., Rui, B., Khan, J., Datta, I. & Giri, S. An emerging potential of metabolomics in multiple sclerosis: a comprehensive overview. Cell Mol. Life Sci. 78, 3181–3203 (2021).
Ganz, T. et al. Serum asymmetric dimethylarginine and arginine levels predict microvascular and macrovascular complications in type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 33, e2836 (2017).
Moore, S. C. et al. Human metabolic correlates of body mass index. Metabolomics 10, 259–269 (2014).
Song, J. et al. The relationship between body mass index and bone mineral density: a mendelian randomization study. Calcif. Tissue Int. 107, 440–445 (2020).
Skaaby, T. et al. Estimating the causal effect of body mass index on hay fever, asthma and lung function using Mendelian randomization. Allergy 73, 153–164 (2018).
Yang, X.-L. et al. Causal link between lipid profile and bone mineral density: a Mendelian randomization study. Bone 127, 37–43 (2019).
Grotzinger, A. D. et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat. Hum. Behav. 3, 513–525 (2019).
Demange, P. A. et al. Investigating the genetic architecture of noncognitive skills using GWAS-by-subtraction. Nat. Genet. 53, 35–44 (2021).
Lu, T., Forgetta, V., Greenwood, C. M. T. & Richards, J. B. Identifying causes of fracture beyond bone mineral density: evidence from human genetics. J. Bone Min. Res. https://doi.org/10.1002/jbmr.4632 (2022).
Nethander, M. et al. BMD-related genetic risk scores predict site-specific fractures as well as trabecular and cortical bone microstructure. J. Clin. Endocrinol. Metab. 105, e1344–e1357 (2020).
Heemskerk, M. M., van Harmelen, V. J., van Dijk, K. W. & van Klinken, J. B. Reanalysis of mGWAS results and in vitro validation show that lactate dehydrogenase interacts with branched-chain amino acid metabolism. Eur. J. Hum. Genet. 24, 142–145 (2016).
Kanno, T. et al. Lactate dehydrogenase M-subunit deficiency: a new type of hereditary exertional myopathy. Clin. Chim. Acta 173, 89–98 (1988).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389 (2017).
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
Taliun, D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021).
Forgetta, V. et al. Cohort profile: genomic data for 26 622 individuals from the Canadian Longitudinal Study on Aging (CLSA). BMJ Open 12, e059021 (2022).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Jiang, L. et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat. Genet. 51, 1749–1755 (2019).
Wang, H. et al. Genotype-by-environment interactions inferred from genetic effects on phenotypic variability in the UK Biobank. Sci. Adv. 5, eaaw3538 (2019).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Yang, J. et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 43, 519–525 (2011).
Visscher, P. M. et al. Statistical power to detect genetic (co)variance of complex traits using SNP data in unrelated samples. PLoS Genet. 10, e1004269 (2014).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Stacey, D. et al. ProGeM: a framework for the prioritization of candidate causal genes at molecular quantitative trait loci. Nucleic Acids Res. 47, e3 (2019).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. JAMA 318, 1925 (2017).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 375, n2233 (2021).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization. J. Am. Med. Assoc. 326, 1614 (2021).
Dendrou, C. A., Petersen, J., Rossjohn, J. & Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339 (2018).
Forgetta, V. Snappy: A flexible SNP proxy finder. https://doi.org/10.5281/zenodo.7328428 (2022).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Richardslab/metabolomics_GWAS_CLSA: CLSA_metabolomics_GWAS_codes. https://doi.org/10.5281/zenodo.7331471 (2022).
Acknowledgements
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation, and the following provinces, Newfoundland, Nova Scotia, Quebec, Ontario, Manitoba, Alberta and British Columbia. This research has been conducted using the CLSA Metabolomics data (v1), CLSA Baseline dataset (v5) and Genomics data (v3), under application number 2006016. The CLSA is led by Drs. Parminder Raina, Christina Wolfson and Susan Kirkland. We also acknowledge the effort of the International Parkinson’s Disease Genomics Consortium (IPDGC), the International Multiple Sclerosis Genetics Consortium (IMSGC), the Diabetes Genetics Replication And Meta-analysis (DIAGRAM) consortium and the MEGASTROKE consortium in providing high-quality GWASs data for other researchers.
The Richards research group is supported by the Canadian Institutes of Health Research (CIHR: 365825, 409511 and 100558), the Lady Davis Institute of the Jewish General Hospital, the Canadian Foundation for Innovation, the NIH Foundation, Cancer Research UK (grant number C18281/A29019), Genome Québec, the Public Health Agency of Canada, Genome Québec, McGill University and the Fonds de Recherche Québec Santé (FRQS). J.B.R. is supported by an FRQS Clinical Research Scholarship. Support from Calcul Québec and Compute Canada is acknowledged. TwinsUK is funded by the Welcome Trust, Medical Research Council, European Union, the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Center based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. These funding agencies had no role in the design, implementation or interpretation of this study. Y.C. has been supported by an FRQS. doctoral training fellowship and the Lady Davis Institute/TD Bank Studentship Award. T.L. has been supported by a Vanier Canada Graduate Scholarship, an FRQS. doctoral training fellowship and a McGill University Faculty of Medicine studentship. T.N. is supported by a research fellowship of the Japan Society for the Promotion of Science for Young Scientists. C.-Y.S. has been supported by the Lady Davis Institute/TD Bank Studentship Award. S.Z. has been supported by a CIHR fellowship and an FRQS. fellowship. P.R. holds the Raymond and Margaret Labarge Chair in Optimal Aging and Knowledge Application for Optimal Aging, is the Director of the McMaster Institute for Research on Aging and the Labarge Center for Mobility in Aging and holds a Tier 1 Canada Research Chair in Geroscience.
Author information
Authors and Affiliations
Contributions
Conception and design: Y.C., V.F., S.Z. and J.B.R. Methodology: Y.C., T.L., Y.F., V.F., C.O., S.Z. and J.B.R. Data curation: Y.C. Data Analysis: Y.C., T.L. and U.P.-K. Visualization: Y.C. and T.L. Writing—Original Draft: Y.C. Writing—Review and Editing: Y.C., T.L., U.P.-K., I.D.S., G.B.-L., T.N., A.C., K.Y.H.L., S.Y., J.D.S.W., C.-Y.S., P.R., C.O., C.M.T.G., Y.F., V.F., C.L., S.Z. and J.B.R. Supervision: J.B.R. Project administration: J.B.R. Funding acquisition: J.B.R.
Corresponding author
Ethics declarations
Ethics declarations
This study was approved by the research ethics boards of the Jewish General Hospital, protocol number 2021–2762.
Competing interests
J.B.R. has served as an advisor to GlaxoSmithKline and Deerfield Capital. J.B.R.’s institution has received investigator-initiated grant funding from Eli Lilly, GlaxoSmithKline and Biogen for projects unrelated to this research. J.B.R. is the CEO of 5 Prime Sciences (http://www.5primesciences.com). T.L. and V.F. are employees of 5 Prime Sciences. Y.F. consults for Fulcrum Genomics, 5 Prime Sciences, and Demetria. T.N. has received speaking fees from Boehringer Ingelheim and AstraZeneca. All other authors declare that there are no conflicts of interest. The opinions expressed in this manuscript are the authors’ own and do not reflect the views of the Canadian Longitudinal Study on Aging.
Peer review
Peer review information
Nature Genetics thanks Amalio Telenti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes and Supplementary Figs. 1–6.
Supplementary Tables
Supplementary Tables 1–26.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Lu, T., Pettersson-Kymmer, U. et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet 55, 44–53 (2023). https://doi.org/10.1038/s41588-022-01270-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-022-01270-1
This article is cited by
-
Unraveling the causality between chronic obstructive pulmonary disease and its common comorbidities using bidirectional Mendelian randomization
European Journal of Medical Research (2024)
-
Plasma metabolites and risk of seven cancers: a two-sample Mendelian randomization study among European descendants
BMC Medicine (2024)
-
Investigating genetic links between blood metabolites and preeclampsia
BMC Women's Health (2024)
-
Exploring the causal role of multiple metabolites on ovarian cancer: a two sample Mendelian randomization study
Journal of Ovarian Research (2024)
-
Plasma campesterol and ABCG5/ABCG8 gene loci on the risk of cholelithiasis and cholecystitis: evidence from Mendelian randomization and colocalization analyses
Human Genomics (2024)