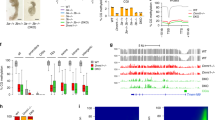
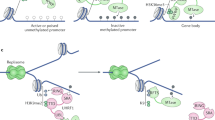
Abstract
In mammals, DNA 5-hydroxymethylcytosine (5hmC) is involved in methylation reprogramming during early embryonic development. Yet, to what extent 5hmC participates in genome-wide methylation reprogramming remains largely unknown. Here, we characterize the 5hmC landscapes in mouse early embryos and germ cells with parental allele specificity. DNA hydroxymethylation was most strongly correlated with DNA demethylation as compared with de novo or maintenance methylation in zygotes, while 5hmC was targeted to particular de novo methylated sites in postimplantation epiblasts. Surprisingly, DNA replication was also required for 5hmC generation, especially in the female pronucleus. More strikingly, aberrant nuclear localization of Dnmt1/Uhrf1 in mouse zygotes due to maternal deficiency of Nlrp14 led to defects in DNA-replication-coupled passive demethylation and impaired 5hmC deposition, revealing the divergency between genome-wide 5-methylcytosine (5mC) maintenance and Tet-mediated oxidation. In summary, our work provides insights and a valuable resource for the study of epigenetic regulation in early embryo development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw sequencing data generated in this study and the corresponding processed files are deposited in the NCBI GEO database under the accession number GSE186357. The following datasets included in this study are available via the NCBI GEO database or the European Nucleotide Archive: scCOOL-seq data of mouse oocytes, sperms, blastocyst (GSE78140); NOMe-seq data of mouse epiblast (GSE79552); WGBS of data WT and Tet-TKO E6.5 epiblast (GSE76261); WGBS data of mouse E6.5 epiblast and PGCs (ERP001953); scRNA-seq data of mouse oocytes (GSE114822); scRNA-seq data of mouse early embryos (GSE45719). Also, mouse gametes and early embryonic dataset with allele-specific peaks of H3K4me3 (GSE71434), H3K9me2 (GSE112320), H3K9me3 (GSE97778), H3K27me3 (GSE76687) and H3K36me3 (GSE112835) modifications. Source data are provided with this paper.
Code availability
This study did not generate any unique code or algorithm. The algorithms used for the analysis during this study are all publicly available. All code supporting this study is available from the corresponding author upon reasonable request.
References
Gu, T. P. et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477, 606–610 (2011).
Wossidlo, M. et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat. Commun. 2, 241 (2011).
Iqbal, K., Jin, S. G., Pfeifer, G. P. & Szabo, P. E. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl Acad. Sci. USA 108, 3642–3647 (2011).
Hackett, J. A. et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 339, 448–452 (2013).
Dawlaty, M. M. et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell 24, 310–323 (2013).
Yamaguchi, S., Shen, L., Liu, Y., Sendler, D. & Zhang, Y. Role of Tet1 in erasure of genomic imprinting. Nature 504, 460–464 (2013).
Yamaguchi, S. et al. Tet1 controls meiosis by regulating meiotic gene expression. Nature 492, 443–447 (2012).
Inoue, A. & Zhang, Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science 334, 194 (2011).
Amouroux, R. et al. De novo DNA methylation drives 5hmC accumulation in mouse zygotes. Nat. Cell Biol. 18, 225–233 (2016).
Hirasawa, R. et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 22, 1607–1616 (2008).
Guo, F. et al. Active and passive demethylation of male and female pronuclear DNA in the mammalian zygote. Cell Stem Cell 15, 447–459 (2014).
Shen, L. et al. Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell Stem Cell 15, 459–471 (2014).
Peat, J. R. et al. Genome-wide bisulfite sequencing in zygotes identifies demethylation targets and maps the contribution of TET3 oxidation. Cell Rep. 9, 1990–2000 (2014).
Nakamura, T. et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature 486, 415–419 (2012).
Begemann, M. et al. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J. Med. Genet. 55, 497–504 (2018).
Hui, P., Buza, N., Murphy, K. M. & Ronnett, B. M. Hydatidiform moles: genetic basis and precision diagnosis. Annu. Rev. Pathol. 12, 449–485 (2017).
Docherty, L. E. et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat. Commun. 6, 8086 (2015).
Murdoch, S. et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat. Genet. 38, 300–302 (2006).
Amoushahi, M., Sunde, L. & Lykke-Hartmann, K. The pivotal roles of the NOD-like receptors with a PYD domain, NLRPs, in oocytes and early embryo development. Biol. Reprod. 101, 284–296 (2019).
Qin, D. et al. The subcortical maternal complex protein Nlrp4f is involved in cytoplasmic lattice formation and organelle distribution. Development 146, dev183616 (2019).
Tong, Z. B. et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 26, 267–268 (2000).
Mahadevan, S. et al. Maternally expressed NLRP2 links the subcortical maternal complex (SCMC) to fertility, embryogenesis and epigenetic reprogramming. Sci. Rep. 7, 44667 (2017).
Schutsky, E. K. et al. Nondestructive, base-resolution sequencing of 5-hydroxymethylcytosine using a DNA deaminase. Nat. Biotechnol. https://doi.org/10.1038/nbt.4204 (2018).
Guo, F. et al. Single-cell multi-omics sequencing of mouse early embryos and embryonic stem cells. Cell Res. 27, 967–988 (2017).
Hackett, J. A., Zylicz, J. J. & Surani, M. A. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 28, 164–174 (2012).
Seisenberger, S., Peat, J. R. & Reik, W. Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells. Curr. Opin. Cell Biol. 25, 281–288 (2013).
Seisenberger, S. et al. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 48, 849–862 (2012).
Li, X. et al. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev. Cell 15, 547–557 (2008).
Strogantsev, R. et al. Allele-specific binding of ZFP57 in the epigenetic regulation of imprinted and non-imprinted monoallelic expression. Genome Biol. 16, 112 (2015).
Au Yeung, W. K. et al. Histone H3K9 methyltransferase G9a in oocytes is essential for preimplantation development but dispensable for CG methylation protection. Cell Rep. 27, 282–293 e284 (2019).
Zeng, T. B., Han, L., Pierce, N., Pfeifer, G. P. & Szabo, P. E. EHMT2 and SETDB1 protect the maternal pronucleus from 5mC oxidation. Proc. Natl Acad. Sci. USA 116, 10834–10841 (2019).
Dai, H. Q. et al. TET-mediated DNA demethylation controls gastrulation by regulating Lefty-Nodal signalling. Nature 538, 528–532 (2016).
Guo, H. et al. DNA methylation and chromatin accessibility profiling of mouse and human fetal germ cells. Cell Res. 27, 165–183 (2017).
Shahbazi, M. N., Siggia, E. D. & Zernicka-Goetz, M. Self-organization of stem cells into embryos: a window on early mammalian development. Science 364, 948–951 (2019).
Yan, R. et al. Decoding dynamic epigenetic landscapes in human oocytes using single-cell multi-omics sequencing. Cell Stem Cell 28, 1641–1656.e7 (2021).
Gu, C., Liu, S., Wu, Q., Zhang, L. & Guo, F. Integrative single-cell analysis of transcriptome, DNA methylome and chromatin accessibility in mouse oocytes. Cell Res. 29, 110–123 (2019).
Wu, S. C. & Zhang, Y. Active DNA demethylation: many roads lead to Rome. Nat. Rev. Mol. Cell Biol. 11, 607–620 (2010).
Barlow, D. P. Genomic imprinting: a mammalian epigenetic discovery model. Annu. Rev. Genet. 45, 379–403 (2011).
Hackett, J. A. & Surani, M. A. DNA methylation dynamics during the mammalian life cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20110328 (2013).
Szabo, P. E., Hubner, K., Scholer, H. & Mann, J. R. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech. Dev. 115, 157–160 (2002).
Smallwood, S. A. et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat. Methods 11, 817–820 (2014).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Shen, Y. et al. A map of the cis-regulatory sequences in the mouse genome. Nature 488, 116–120 (2012).
Acknowledgements
We thank G.-L. Xu (Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China) for providing Tet3-related materials, including Tet triple KO embryonic stem cells, Tet3 conditional KO mice and related antibodies. We also thank S. Li (Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China) and the imaging platform of CAS for the support with the fluorescence immunoassay. The National Supercomputer in Guangzhou (TianHe-2) supported the bioinformatic analysis of this study. This study was supported by grants from the National Key Research and Development Program of China (grant no. 2018YFA0107701 to F.G., grant no. 2020YFA0112201 to H.W., grant no. 2021YFC2700302 to H.W.), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDA16021400 to F.G.) and the CAS Project for Young Scientists in Basic Research (grant no. YSBR-012 to F.G.).
Author information
Authors and Affiliations
Contributions
F.G. conceived this project and together with H.W. supervised the study. R.Y., X.C., Y.X., J.Z., F.S., J.Q. and Y.D. performed the experiments. C.G. and X.L. analyzed the bioinformatic data. F.G. wrote the manuscript with help from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Assessment of Tet3 and DNA replication in affecting 5hmC generation.
a, Immunostaining showed the absence of Tet3 protein in Tet3 maternal KO zygotes. b, Immunostaining showed a reduction in 5hmC signals in the male pronucleus in Tet3 maternal KO zygotes. c, Immunostaining showed the prevention of EdU incorporation into DNA in zygotes after treated with aphidicolin. d, Immunostaining showed 5hmC signals in aphidicolin-treated zygotes. e, Bar plot showing the number of significant 5hmCpG sites against covered CpG sites at different genomic elements in gametes and preimplantation embryos. ‘2C to blast. demeth.’ denotes the DNA demethylation regions from the 2-cell stage to the blastocyst stage. Oocytes, n = 2; Sperm, n = 2; 2-Cell, n = 2; 4-Cell, n = 3; 8-Cell, n = 2; Blastocyst, n = 5. f, Venn plot showing the overlap of demethylation regions identified in male and female pronucleus in Extended Data Fig. 2b. g, Bar plot showing the number of Tet3-dependent and DNA replication-coupled 5hmC loci in the pronucleus. h, Venn plot showing the overlap of Tet3-dependent 5hmC loci in male and female pronucleus. i, j, Venn plots showing the overlap of Tet3-dependent and DNA replication-coupled 5hmC loci in male (i) and female pronucleus (j). k, l, Pie charts showing the distribution of Tet3-dependent 5hmC loci (k) and DNA replication-coupled 5hmC loci (l) in female pronucleus on different genomic elements. m, Representative Tet3-dependent and DNA replication-coupled 5hmC loci in the mouse pronucleus. Representative images from at least three independent biological replicates were showed in Extended Data Fig. 1a–d.
Extended Data Fig. 2 The relationship of 5hmC generation with demethylation and de novo methylation in mouse zygotes.
a, Ternary plots showing the levels of cytosine, 5mC and 5hmC in 1-kb bins across the genome. b, Heat maps showing the dynamics of 5mC level and 5hmC level in mouse sperm to male pronucleus (left panel) and oocytes to female pronucleus (right panel). Four groups of 5mC regions were identified based on the changes in DNA methylation. c,d, Violin plots showing the 5mC levels and 5hmC levels in mouse sperm and male pronucleus (c) or oocytes and female pronucleus (d) on four groups of 5mC regions. e, f, Density plots showing the intensity of 5mC level (left panel) and 5hmC level (right panel) on demethylated regions (upper panel) / de novo methylated regions (lower panel) in mouse sperm and male pronucleus (e) or oocytes and female pronucleus (f). g, h, Box plots showing the 5mC level (g) or 5hmC level (h) of demethylated regions, de novo methylated regions and random selected tiles in sperm and male pronucleus. i,j, Box plots showing the 5mC level (i) or 5hmC level (j) of demethylated regions, de novo methylated regions and random selected tiles in oocytes and female pronucleus.
Extended Data Fig. 3 Examination the relationship between demethylation or de novo methylation and 5hmC in germ cell development.
a, Average DNA methylation levels around genebodies (±2 kb) in mouse epiblasts and germline cells. b, DNA methylation levels and 5hmC levels in mouse epiblasts and germline cells. c, Density plots showing the intensity of 5mC level (left panel) and 5hmC levels (right panel) on demethylated regions in mouse E6.5 epiblast and E9.5 PGCs. d, Bar plots showing the 5mC level or 5hmC level of demethylated regions and random selected regions from E6.5 epiblast to E9.5 PGCs. e, Density plots showing the intensity of 5mC level and 5hmC levels on demethylated regions in mouse E9.5 and E11.5 PGCs. f, Bar plots showing the 5mC level or 5hmC level of demethylated regions and random selected regions from E9.5 to E11.5 PGCs. g, Density plots showing the intensity of 5mC level and 5hmC level in mouse E13.5 and E16.5 male germ cells. h, Box plots showing the 5mC level or 5hmC level of de novo methylated regions and random selected regions in E13.5 and E16.5 male germ cells. i, Density plots showing the intensity of 5mC level and 5hmC level in mouse E16.5 male germ cells and sperm. j, Box plots showing the 5mC level or 5hmC level of de novo methylated regions and random selected regions in E16.5 male germ cells and sperm. k, Density plots showing the intensity of 5mC level and 5hmC level in mouse E16.5 female germ cells and oocytes. l, Box plots showing the 5mC level or 5hmC level of de novo methylated regions and random selected regions in E16.5 female germ cells and oocytes.
Extended Data Fig. 4 Discrimination of parental genomes by SNP information.
a, Percentage of allele-specific reads derived from maternal or paternal genomes across developmental stages. b, Number of significant 5hmCpG sites against covered CpG sites derived from the maternal or paternal genome across developmental stages. c, Number of detected counts derived from the maternal or paternal genome across developmental stages. d, Number of detected genes (TPM ≥ 0.1) derived from the maternal or paternal genome across developmental stages. e, Expression levels of DNA (de)methylation related genes from the maternal and paternal genomes across developmental stages. f, The dynamics of 5hmC levels from the maternal and paternal genomes across developmental stages. g, Representative 5hmCpG loci in the parental genomes across developmental stages.
Extended Data Fig. 5 Dynamics of Tet3-dependent hyper-hmDMRs across preimplantation development.
a,b, Dynamics of 5hmC levels (a) and 5mC levels (b) of Tet3-dependent hyper-hmDMRs identified in male pronucleus from gametes to pre-implantation embryos. c, d, Dynamics of 5hmC levels (c) and 5mC levels (d) of Tet3-dependent hyper-hmDMRs identified in female pronucleus from gametes to pre-implantation embryos. e, Pie chart showing the proportion of paternal stable 5hmC regions in Tet3-dependent hyper-hmDMRs in male pronucleus. f, Representative Gene Ontology (GO) terms for paternal stable 5hmC region related genes. g, Pie chart showing the proportion of maternal stable 5hmC regions in Tet3-dependent hyper-hmDMRs in female pronucleus. h, Representative Gene Ontology (GO) terms for maternal stable 5hmC regions related genes. i,j, Bar plots showing the expression level of paternal (i) or maternal (j) genes related with stable 5hmC regions in pre-implantation embryos. The paternal (or maternal) stable 5hmC regions were defined from Tet3-dependent hyper-hmDMRs in male (or female) pronucleus, in which 5hmC level from paternal (or maternal) genome at blastocyst stage were still higher than 5%, and the difference for 5hmC level between male (or female) pronucleus and the paternal (or maternal) genome in blastocyst is less than 5%.
Extended Data Fig. 6 The relationship of 5hmC with gene expression.
a, Number of significant 5hmCpG sites against covered CpG sites in maternal- and paternal-activated genes across developmental stages. b, Relative enrichment of the Tet3-dependent hyper-hmDMRs in male pronucleus or hyper-hmDMRs in paternal 2-cell embryos on paternal activated genes and random selected genes. c, Relative enrichment of the Tet3-dependent hyper-hmDMRs in male pronucleus or hyper-hmDMRs in paternal 2-cell embryos on different genomic elements. d, Number of significant 5hmCpG sites against covered CpG sites in genomic elements of the parental genomes. e, f, Box plots showing the expression level of genes with 5hmC-CGI promoters identified in female (e) or male (f) pronucleus across development stages. g, Box plot showing the expression level of genes with 5hmC-CGI promoters in E6.5 epiblast and germ cells. To identify the genes with 5hmC-CGI promoters, high 5hmC fraction tiles were overlapped with CGI-promoters in each stage, respectively. The genes which CGI-promoters overlapped with high 5hmC fraction tiles were identified as 5hmC-CGI genes. h,i, Ternary plots showing the levels of cytosine, 5mC and 5hmC of maternal genome in H3K9me2 enriched regions identified in oocytes. j,k, Ternary plots showing the levels of cytosine, 5mC and 5hmC of Tet3-dependent-hmDMRs that were overlapped or not-overlapped with H3K9me2 modifications (n = 59). The ChIP-seq data of oocyte H3K9me2 was accessed at GEO (GSE112320).
Extended Data Fig. 7 Enhancer-enriched 5hmC in mouse E6.5 epiblasts linked with developmental genes.
a, Number of hyper-hmDMRs in mouse blastocysts and epiblasts. b, 5hmC level of the hyper-hmDMRs identified in mouse epiblasts. c, Relative enrichment of the hyper-hmDMRs of epiblasts in different genomic elements. d, Expression level of hmDMR genes and pluripotency-related genes. e, Representative Gene Ontology (GO) terms for hmDMR genes. f, Representative loci of hyper-hmDMRs identified in epiblasts. g, DNA methylation level of hyper-hmDMRs in mouse epiblasts (WT vs. Tet-TKO). h,i, Normalized GCH methylation level in distal NDRs (h) and hyper-hmDMRs (i) in mouse blastocysts and epiblasts. j, Percentage of hyper-hmDMRs with different DNA methylation statuses between WT and Tet-TKO epiblasts. k, Representative 5hmCpG and DNA methylation levels of hyper-hmDMRs in mouse blastocysts and epiblasts.
Extended Data Fig. 8 Examination the relationship between de novo DNA methylation and 5hmC in E6.5 epiblast.
a, Bar plot showing the expression of epiblast genes in Oct4-EGFP-positive cells of IVC embryos (n = 2). b, Bar plot showing the 5mC levels of E6.5 epiblast cells and Oct4-EGFP-positive cells of IVC embryos in different genomic elements. c, Relative enrichment of the hyper-DMRs of epiblasts in different genomic elements. d, Heat maps showing the dynamics of 5mC level and 5hmC level in mouse E3.5 ICM, E6.5 epiblasts and IVC embryos. e, Ternary plots showing the levels of cytosine, 5mC and 5hmC at enhancer, genebody, promoter and SINE elements in E6.5 epiblast cells and IVC epiblasts. f,g, Representative genes with reduced 5hmC level at E6.5 epi. hyper hmDMRs. For genes expression, blastocyst (Blast.), n = 30; E6.5 epiblast (E6.5 epi.), n = 4; IVC epiblast (IVC epi.), n = 3.
Extended Data Fig. 9 Alterations in DNA methylation, chromatin accessibility and gene expression in Nlrp14-maternal-KO embryos.
a, Box plot showing the genome-wide DNA methylation level in WT and Nlrp14-KO single MII oocytes and single blastomeres from WT and Nlrp14 -maternal-KO 2-cell embryos. Red dots denote aggregated data of each sample. b, Box plot showing the percent of maternal or paternal DNA reads distinguished from single blastomeres of WT and maternal-KO 2-cell embryos. c, Box plot showing the DNA methylation level in WT and maternal-KO 2-cell embryos based on the 1-kb tiles of the WCG methylation level. WT Oocytes, n = 18; maternal KO Oocytes, n = 20. WT 2-cell embryos, n = 22; maternal KO 2-cell embryos, n = 20. d, Chromatin accessibility of WT and maternal-KO 2-cell embryos at TSSs (±1 kb). e,f, Scatter plot showing the differentially expressed genes in WT and KO oocytes (e) and WT and maternal KO 2-cell embryos (f). g, Box plot showing the percent of maternal (left panel) and paternal RNA reads (right panel) distinguished from single oocytes (WT, n = 14; KO, n = 19) or single embryos (WT, n = 8; KO, n = 9). h,i, Box plots showing the gene expression level of maternal up-regulated genes (h) and down-regulated genes (i) identified in Nlrp14 maternal KO 2-cell embryos. j,k,l, Representative Gene Ontology (GO) terms for maternal up-regulated genes (j), maternal down-regulated genes (k) and paternal downregulated genes (l) identified in mat KO 2-cell embryos.
Extended Data Fig. 10 Reduced global level of 5hmCs in the absence of Nlrp14 in mouse early embryos.
a, DNA methylation level across genebodies (±2 kb) in gametes, WT and maternal KO 2-cell embryos. b, Box plots showing the DNA methylation level of promoters and genebodies in WT and maternal KO 2-cell embryos (left panel). Chromatin accessibility of promoters in WT and maternal-KO 2-cell embryos (right panel). c, Bar plot showing the number of hyper-DMRs identified between WT and maternal KO 2-cell embryos. d, Enrichment analysis of paternal hyper-DMRs detected in maternal-KO 2-cell embryos. e, DNA methylation level of maternal and paternal gDMRs in WT and maternal KO 2-cell embryos. f, Bar plot showing the number of significant 5hmCpG sites on different genomic elements in WT and maternal KO 2-cell embryos. g, The number of significant 5hmCpG sites on ZGA genes between WT and maternal KO 2-cell embryos. h, Representative loci of 5hmCpG, WCG and GCH sites in WT and maternal KO 2-cell embryos.
Supplementary information
Supplementary Information
Supplementary notes, Supplementary Figs. 1–4, legends of supplementary tables and Source data figure.
Supplementary Table 1
Tet3-dependent 5hmC loci in male pronucleus. Tet3-dependent 5hmC loci were defined as hypo-hmDMRs in Tet3Δ male pronucleus when compared with WT male pronucleus. This table includes the following information about these loci: position on genome, 5hmC level in sperm and male pronucleus, length, number of detected CpG sites and genes within 10 kb.
Supplementary Table 2
DNA-replication-coupled 5hmC loci in male pronucleus. DNA-replication-coupled 5hmC loci were defined as hypo-hmDMRs in DNA-replication-inhibited male pronucleus when compared with WT male pronucleus. This table includes the following information about these loci: position on genome, 5hmC level in sperm and male pronucleus, length, number of detected CpG sites and genes within 10 kb.
Supplementary Table 3
Tet3-dependent 5hmC loci in female pronucleus. Tet3-dependent 5hmC loci were defined as hypo-hmDMRs in Tet3Δ female pronucleus when compared with WT female pronucleus. This table includes the following information about these loci: position on genome, 5hmC level in sperm and male pronucleus, length, number of detected CpG sites and genes within 10 kb.
Supplementary Table 4
DNA-replication-coupled 5hmC loci in female pronucleus. DNA-replication-coupled 5hmC loci were defined as hypo-hmDMRs in DNA-replication-inhibited female pronucleus when compared with WT female pronucleus. This table includes the following information about these loci: position on genome, 5hmC level in sperm and male pronucleus, length, number of detected CpG sites and genes within 10 kb.
Supplementary Table 5
Identification of demethylated regions, stable high/low methylation regions, de novo methylated regions between sperm and male pronucleus. This table includes the following information about these regions: position on genome, 5mC and 5hmC level in sperm and male pronucleus, length and genes within 10 kb.
Supplementary Table 6
Identification of demethylated regions, stable high/low methylation regions, de novo methylated regions between oocytes and female pronucleus. This table includes the following information about these regions: position on genome, 5mC and 5hmC level in oocytes and female pronucleus, length and genes within 10 kb.
Source data
Source Data Fig. 1
Gating strategy for fluorescence-activated cell sorting (FACS) of Oct4-EGFP-positive cells. B6;CBA-Tg(Pou5f1-EGFP)2Mnn/J mice were mated with either C57BL/6 or PWK mice. Then, EGFP-positive embryos were digested for FACS to collect mouse E6.5 epiblast cells and PGCs. The gating strategy for cell sorting of E9.5 PGCs is showed in Source Data Fig. 1a. Source Data Fig. 1b shows the gating strategy for the other samples. Firstly, FSC-A × SSC-A were used to exclude debris. Then, SSC-A/SSC-H × SSC-W and FSC-A/FSC-H × FSC-W were used to remove doublets and clumps. Cells in P1–4 or P4 (only for E9.5 PGCs) were collected as EGFP-positive cells; the value on each plot indicates the percentage of positive cells as shown in Source Data Fig. 1c. We used BD Fusion for sorting and BD FACSDiva 8.0.1 for analysis of E9.5 PGCs. BD FACSMelody sorters and BD FACSChorus software were used to collect the other samples.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, R., Cheng, X., Gu, C. et al. Dynamics of DNA hydroxymethylation and methylation during mouse embryonic and germline development. Nat Genet 55, 130–143 (2023). https://doi.org/10.1038/s41588-022-01258-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-022-01258-x
This article is cited by
-
Single-cell profiling reveals transcriptome dynamics during bovine oocyte growth
BMC Genomics (2024)
-
Catalytic-dependent and -independent roles of TET3 in the regulation of specific genetic programs during neuroectoderm specification
Communications Biology (2024)
-
Auto-suppression of Tet dioxygenases protects the mouse oocyte genome from oxidative demethylation
Nature Structural & Molecular Biology (2024)
-
DNA methylation in poultry: a review
Journal of Animal Science and Biotechnology (2023)
-
Loss of the Maternal Effect Gene Nlrp2 Alters the Transcriptome of Ovulated Mouse Oocytes and Impacts Expression of Histone Demethylase KDM1B
Reproductive Sciences (2023)