Abstract
To capture the full spectrum of genetic risk for autism, we performed a two-stage analysis of rare de novo and inherited coding variants in 42,607 autism cases, including 35,130 new cases recruited online by SPARK. We identified 60 genes with exome-wide significance (P < 2.5 × 10−6), including five new risk genes (NAV3, ITSN1, MARK2, SCAF1 and HNRNPUL2). The association of NAV3 with autism risk is primarily driven by rare inherited loss-of-function (LoF) variants, with an estimated relative risk of 4, consistent with moderate effect. Autistic individuals with LoF variants in the four moderate-risk genes (NAV3, ITSN1, SCAF1 and HNRNPUL2; n = 95) have less cognitive impairment than 129 autistic individuals with LoF variants in highly penetrant genes (CHD8, SCN2A, ADNP, FOXP1 and SHANK3) (59% vs 88%, P = 1.9 × 10−6). Power calculations suggest that much larger numbers of autism cases are needed to identify additional moderate-risk genes.
Similar content being viewed by others
Main
Many previous genetic studies in autism spectrum disorder (ASD), a neurodevelopmental condition characterized by social communication difficulties and repetitive behaviors1, focused on de novo variants (DNVs) identified from parent–offspring trios2,3,4,5,6,7,8. Over 100 high-confidence ASD genes enriched with likely deleterious DNVs have been identified8, most of which are also enriched for DNVs in other neurodevelopmental disorders (NDDs)9,10,11. Statistical modeling suggests that there are ~1,000 genes with DNVs in ASD12,13. However, despite the large effect size of individual pathogenic DNVs, all DNVs combined explain only ~2% of variance in liability for ASD8,14. ASD is highly heritable14,15,16, and previous studies estimated that common variants explain up to half of the heritability14, although only five genome-wide significant loci have been identified17. Rare LoF variants in genes intolerant of variation9,18 are overtransmitted to probands compared with siblings without ASD7,8,19,20,21,22. However, identification of the individual risk genes enriched by such inherited variants has remained elusive. We have established the largest ASD cohort, Simons Foundation Powering Autism Research for Knowledge (SPARK)23, which currently includes over 100,000 people with ASD, to advance research on the genetic, behavioral and clinical features associated with ASD.
Rare LoF variants are enriched in developmental disorders including ASD22,24, but may also result from sequencing and annotation artifacts25 and present technical challenges in large sequencing studies. Methods to distinguish between high-confidence and low-confidence LoF variants18,26,27 have been used to quantify gene-level LoF intolerance18,26,28,29 and to refine the role of LoF DNVs in NDDs20.
Here, we present an integrated analysis of de novo and inherited coding variants in over 42,607 ASD cases, including cases from previously published ASD cohorts and 35,130 new cases from SPARK. In our two-stage design, we first characterized the contribution of DNVs and rare inherited LoF variants to ASD risk. Results from the first stage informed the second stage meta-analysis of 404 genes. By combining evidence from DNVs, transmission disequilibrium tests (TDTs) and case-control comparisons, we identified 60 ASD risk genes with exome-wide significance, including five new genes not previously implicated in NDDs. Finally, we estimated the effect sizes of known and newly identified genes and conducted power calculations to inform the design of future studies.
Results
Overview of data and workflow
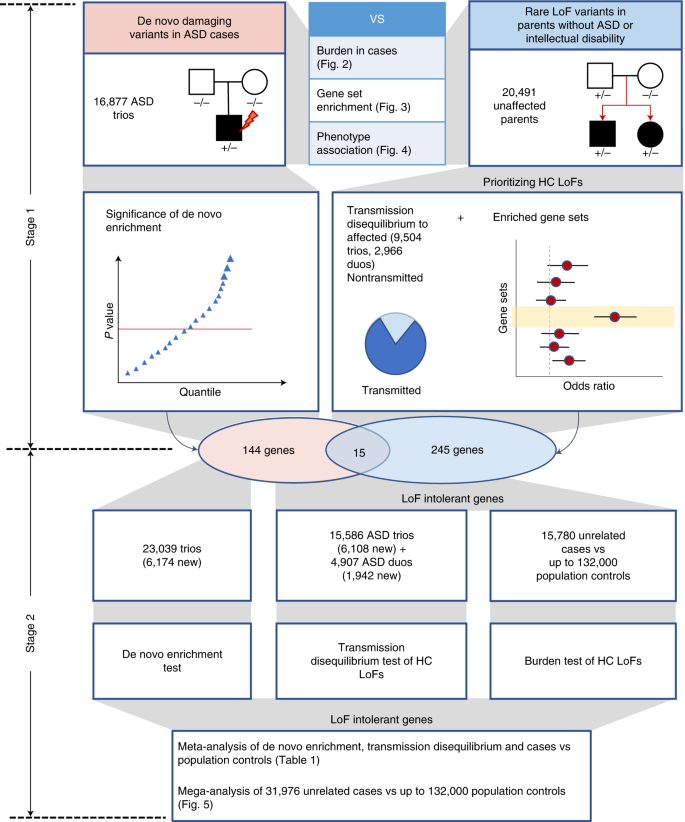
We aggregated exome or whole genome sequencing (WGS) data of 35,130 new cases from SPARK and 7,665 cases from published ASD studies (ASC3,8, MSSNG6 and SSC2,30) (Supplementary Table 1) and performed a two-stage analysis (Fig. 1). In stage 1, we analyzed DNVs in 16,877 ASD trios and assessed transmission of rare LoF variants from 20,491 parents without ASD diagnoses or intellectual disability to offspring with ASD (including 9,504 trios and 2,966 single-parent-proband duos). For DNVs, we characterized the enrichment pattern in known and candidate risk genes, as well as mutation intolerance (probability of being LoF intolerant as defined by the Exome Aggregation Consortium (ExAC pLI)18, and Genome Aggregation Database (gnomAD) metrics26), and performed gene-based burden tests of LoF and missense DNVs by DeNovoWEST11. For rare inherited LoFs, we estimated the overtransmission from unaffected parents to ASD offspring in all genes and gene sets predefined by functional genomic data or results from DNV analysis. Based on DNV enrichment and overtransmission patterns in gene sets, we selected 404 genes for meta-analysis in stage 2 using 22,764 new cases with exome or WGS data. In stage 2, we applied DeNovoWEST on DNVs, conducted TDTs on inherited LoFs in trios or duos, performed burden tests on rare LoFs in unrelated cases compared with population controls (104,068 subjects from non-neuro gnomAD exomes and 132,345 TOPMed subjects) and combined the P values to estimate a final P value for each of the 404 genes. Finally, we performed a mega-analysis of rare LoFs in all cases and controls to estimate the effect sizes of known or new candidate ASD genes to inform future studies.
In the discovery stage, we identified DNVs in 16,877 ASD trios and rare LoF variants in 20,491 parents without ASD diagnoses and intellectual disability. We compared properties of de novo and rare variants to identify rare LoFs that contribute to genetic risk in individuals with ASD. We also evaluated their associations with cognitive impairment and enriched gene sets. We performed an initial exome-wide scan of genes enriched by DNVs or showing transmission disequilibrium of rare LoFs to affected offspring and selected a total of 404 genes for further replication, including 159 de novo enriched genes and 260 prioritized transmission disequilibrium genes from enriched gene sets (15 genes were in both). In the meta-analysis stage, we first evaluated evidence from de novo enrichment and transmission disequilibrium of rare inherited LoFs in an expanded set of family-based samples including over 6,000 additional ASD trios and around 2,000 additional duos. The DNVs in ASD were combined with those from an additional 31,565 NDD trios to refine the filters of high-confidence LoF variants in de novo LoF enriched genes. We also constructed an independent dataset of LoF variants of unknown inheritance from 15,780 cases that were not used in de novo or transmission analysis. We compared LoF rates in cases with two population-based sets of controls (n = ~104,000 and ~132,000, respectively). For 367 LoF-intolerant genes on autosomes, the final gene-level evidence was obtained by meta-analyzing P values of de novo enrichment, transmission disequilibrium of high-confidence rare inherited LoFs, and comparison of high-confidence LoFs from cases and controls not used in the de novo or transmission analysis. We also performed a mega-analysis that analyzed high-confidence LoFs identified in all 31,976 unrelated ASD cases and compared their rates with population-based controls. HC, high-confidence.
Known ASD or NDD risk genes explain most de novo burden
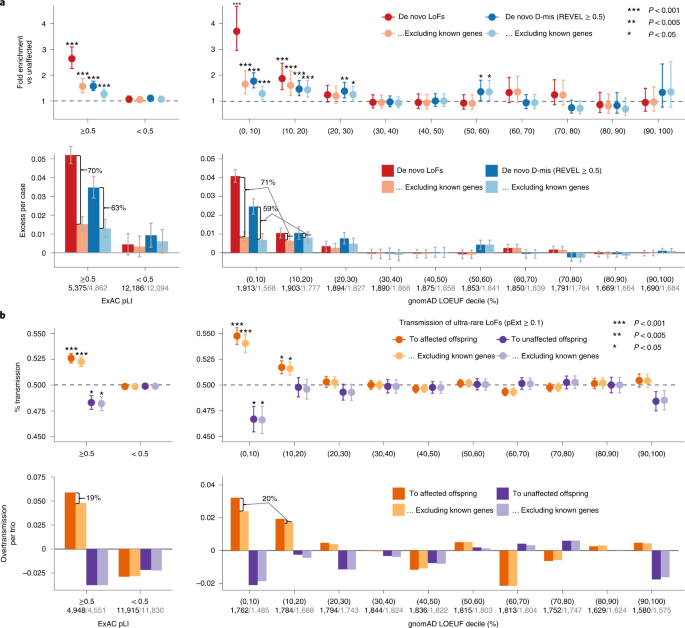
In the first stage, we combined data from four large-scale ASD cohorts, including 16,877 unique ASD trios and 5,764 unaffected trios (Supplementary Table 1). The cohorts show similar exome-wide burden of DNVs in simplex families. The burden of LoF DNVs in cases with an ASD family history is significantly lower than those without (P = 1.1 × 10−4 by Poisson test), whereas the burden of predicted de novo damaging missense (D-mis, defined by rare exome variant ensemble learner (REVEL) score31 ≥ 0.5) and synonymous variants are similar (Extended Data Fig. 1). Compared with unaffected offspring, the excess of damaging DNVs (de novo LoF and D-mis variants) in individuals with ASD is concentrated in LoF-intolerant genes, defined as genes with an ExAC pLI ≥ 0.5 (ref. 18). Using LoF observed/expected upper-bound fraction (LOEUF), a recently developed gene constraint metric26, the burden of damaging DNVs is highest among genes ranked in the top 20% of LOEUF scores (Fig. 2a). Overall, the population attributable risk (PAR) from damaging DNVs is about 10%. We assembled 618 previously established dominant (‘known’) ASD or NDD risk genes (Supplementary Table 2). These genes explained about two-thirds of the PAR from damaging DNVs. Excluding these genes, the fold enrichment of damaging DNVs was greatly attenuated (Fig. 2a).
a, The burden of DNVs was evaluated by the rate ratio and rate difference between 16,877 ASD and 5,764 unaffected trios. The exome-wide burden of de novo LoF and D-mis (REVEL ≥ 0.5) variants are concentrated in constrained genes (ExAC pLI ≥ 0.5) and in genes with the highest levels of LoF intolerance in the population (defined by the top two deciles of gnomAD LOEUF scores). Burden analysis was repeated after removing known ASD or NDD genes. The number of genes before and after removing known genes in each constraint bin is shown below the axis label. Data are presented as mean values and 95% confidence intervals. Among constrained genes (ExAC pLI ≥ 0.5 or the top 20% of gnomAD LOEUF scores), close to two-thirds of case-control rate differences of de novo LoF and D-mis variants can be explained by known genes. Exact P values by Poisson test are listed in Supplementary Table 19. b, The burden of inherited LoFs was evaluated by looking at the proportion of rare LoFs in 20,491 parents without ASD diagnoses or intellectual disability that are transmitted to affected offspring in 9,504 trios and 2,966 duos and show evidence of overtransmission of LoFs per ASD trio. As a comparison, we also show the transmission disequilibrium pattern to unaffected offspring in 5,110 trios and 129 duos. Data are presented as mean values ± standard errors as error bars. Two-sided binomial test was used to compute the P values for overtransmission or undertransmission. Using ultra-rare LoFs with pExt ≥ 0.1, exome-wide signals of transmission disequilibrium of rare inherited LoF variants also concentrate in constrained genes (ExAC pLI ≥ 0.5) and in genes within the top two deciles of gnomAD LOEUF scores. Analysis was restricted to autosomal genes and repeated after removing known ASD or NDD genes (number of genes in each constrained bin before and after removing known genes is shown below the axis label). Among all constrained genes, only one-fifth of overtransmission of LoFs to ASD trios can be explained by known ASD or NDD genes. Exact P values by binominal test are listed in Supplementary Table 19.
To assess the evidence of DNVs in individual genes, we applied DeNovoWEST11, which integrates DNV enrichment with clustering of missense variants in each gene. The initial DeNovoWEST scan of DNVs in 16,877 ASD trios identified 159 genes with P < 0.001 (Supplementary Table 3).
Rare inherited LoFs are mostly in unknown ASD risk genes
To analyze the contribution of rare inherited LoF variants to ASD risk, we evaluated transmission disequilibrium in ultra-rare (allele frequency < 1 × 10−5) high-confidence (by the loss-of-function transcript effect estimator (LOFTEE)26 package and proportion expression across transcripts (pExt)27; see Methods and Supplementary Note) LoF variants from parents without ASD diagnoses or intellectual disability to affected offspring with ASD in 9,504 trios and 2,966 duos from the first stage (Supplementary Table 4). For a given set of genes, we quantified transmission disequilibrium using the number of overtransmitted (excess in transmission over nontransmission) LoF variants per trio; parent–offspring duos were considered half-trios. Among autosomal genes, the overall transmission disequilibrium signal of ultra-rare LoF variants is enriched in LoF-intolerant genes (ExAC pLI ≥ 0.5) and in genes within the top 20% of LOEUF scores (Fig. 2b), similar to the burden of damaging DNVs. We observed both overtransmission to affected and undertransmission to unaffected offspring, especially in genes within the top 10% of LOEUF scores. However, known ASD or NDD genes explain only ~20% of overtransmission of LoF variants to affected offspring (Fig. 2b). On the X chromosome, we only considered transmission from mothers without ASD to 9,883 affected sons and 2,571 affected daughters (Supplementary Table 4). Rare LoF variants in mothers without ASD show significant overtransmission to affected sons but not affected daughters and remain significant after removing known ASD or NDD genes (Supplementary Fig. 1). Together, these results suggest that most genes conferring inherited ASD risk are yet to be identified. Autosomal rare D-mis variants also show evidence of transmission disequilibrium to affected offspring, although the signal is much weaker (Supplementary Fig. 2).
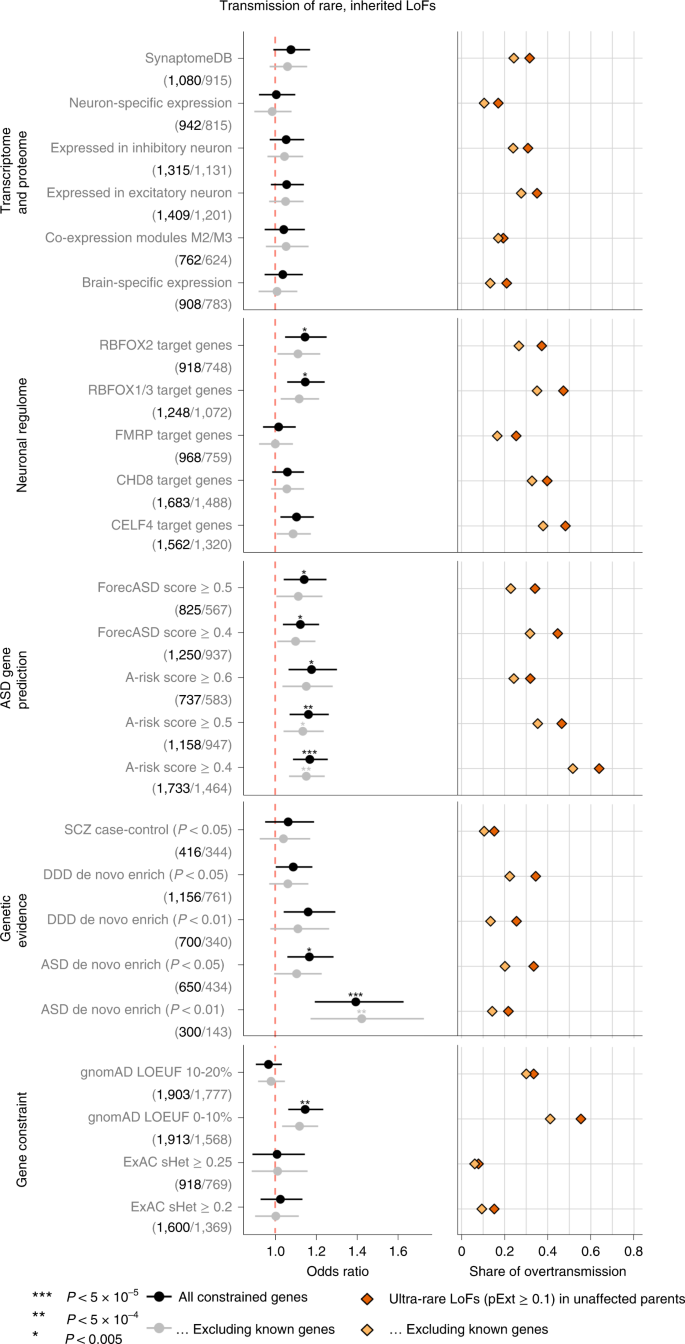
To characterize the properties of genes contributing to ASD risk through rare inherited variants, we defined 25 gene sets from five categories representing both functional and genetic evidence relevant to ASD (Supplementary Table 5 and Supplementary Fig. 3). We limited the genes to 5,754 autosomal constrained genes (ExAC pLI ≥ 0.5 or top 20% of LOEUF scores) and performed TDT (Supplementary Table 6). For each gene set, we tested if ultra-rare high-confidence rare LoF variants show a higher transmission to ASD offspring than the remaining genes in the overall constrained gene set. As a comparison with DNVs, we also tested if the same set of genes are more frequently disrupted by damaging DNVs than the rest of the genes in ASD trios using DNENRICH32.
Using functional gene sets derived from the neuronal transcriptome, proteome or regulome, we confirmed significant enrichment in damaging DNVs (P < 0.005 by simulation) in the gene sets that were previously suggested to be enriched for ASD risk genes including expression module M2/333, RBFOX1/3 targets34, FMRP targets35 and CHD8 targets36. However, this enrichment can be largely explained by known ASD or NDD genes (Extended Data Fig. 2). For ultra-rare inherited LoF variants, we found that the proportion of transmission to ASD individuals in most functional gene sets is close to all other genes; only RBFOX targets show a weak enrichment but can be largely explained by known genes (Fig. 3). We also applied two machine learning methods to prioritize ASD risk genes: forecASD37 and A-risk38. Although enrichment of DNVs in predicted genes is mainly explained by known genes, genes prioritized by A-risk are significantly enriched with inherited LoFs that are not explained by known genes. Using A-risk ≥ 0.4, 30% of constrained genes (n = 1,464) were prioritized and explain 64% of the overtransmission of LoF variants to ASD offspring (P = 2.6 × 10−5 by χ2 test). This enrichment is higher than genes prioritized by the LOEUF score; 33% of genes (n = 1,777) in the top decile of LOEUF account for 55% of the overtransmission (P = 3.5 × 10−4 by χ2 test) (Fig. 3).
Gene sets were defined and grouped by transcriptome proteome, neuronal regulome, ASD gene prediction scores, genetic evidence from neuropsychiatric diseases, and gene-level constraint. Analyses were repeated after removing known ASD or NDD genes. (Number of genes in each set before and after removing known genes are shown in parentheses below gene set.) Dots represent fold enrichment of DNVs or odds ratios for overtransmission of LoF variants in each set. Horizontal bars are presented as mean values with 95% confidence interval as error bars. For each gene set, we show the percentage of overtransmission of rare LoFs to cases. Enrichment of rare inherited LoFs was evaluated by the share of overtransmission events (the transmission and nontransmission of ultra-rare LoFs with pExt ≥ 0.1) in the selected gene set vs those in all other constrained genes using a two-by-two table. P values were determined using the χ2 test. Exact P values are listed in Supplementary Table 19.
We also considered gene sets that have evidence of genetic association with DNVs. Genes nominally enriched by DNVs (P < 0.01 by DeNovoWEST; n = 300) in ASD from the current study have a significantly higher overtransmission rate than other constrained genes (odds ratio = 1.39, P = 3.0 × 10−5 by χ2 test) (Fig. 3), although these genes account for only 21% of the overtransmission. Genes nominally enriched by DNVs in other NDDs11 are also significantly enriched by DNVs in ASD and weakly enriched by inherited LoFs in ASD; however, both can be largely explained by known genes (Fig. 3). This suggests that a subset of ASD genes increase risk by both DNVs and inherited variants, and new genes can be identified by integrating evidence from DNV enrichment and TDT.
DNVs and some rare inherited LoFs are associated with intellectual disability
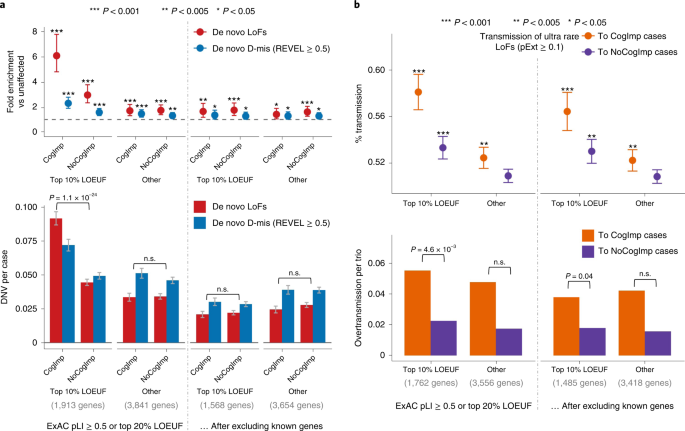
To evaluate the association of genotypes with phenotype in ASD, we used self-reported cognitive impairment in SPARK, a Vineland score of <70 in the SSC or the presence of intellectual disability in ASC. Damaging DNVs in genes ranked within the top 10% of LOEUF scores show a higher burden (P = 1.1 × 10−24 by χ2 test) in ASD cases with evidence of cognitive impairment than in other cases, consistent with previous results2,8 (Fig. 4a). Once known ASD or NDD genes were excluded, the residual burden of damaging DNVs in genes within the top 10% of LOEUF scores is greatly reduced and not significantly associated with cognitive phenotype in ASD (Fig. 4a). Overtransmission of rare LoFs in genes within the top 10% of LOEUF genes to ASD cases with cognitive impairment is about 2.7 times higher than to cases without cognitive impairment (P = 4.6 × 10−3 by χ2 test) and is still 2 times higher (P = 0.04 by χ2 test) once known ASD or NDD genes were excluded (Fig. 4b). However, rare LoFs in genes prioritized by A-risk are not associated with cognitive impairment (Supplementary Fig. 4). Taken together, these results suggest that rare variants in the top 10% of LOEUF genes—most of which are already known to be ASD or NDD risk genes—are associated with cognitive impairment. However, a subset of rare inherited variants, particularly those prioritized by A-risk, are not associated with cognitive impairment.
Ultra-rare inherited LoFs with pExt ≥ 0.1 in genes with the top 10% of gnomAD LOEUF scores also show a higher proportion of transmission and a higher overtransmission rate to ASD offspring with cognitive impairment (CogImp) than those without (NoCogImp). Rare LoFs in other constrained genes are not significantly associated with phenotypic severity. The increased burden of inherited LoFs in cases with cognitive impairment remains significant after removing known ASD or NDD genes. Data are presented as mean values ± standard errors as error bars. Poisson test was used to compute the P values to assess the fold enrichment, and binominal test was used for overtransmission. Exact P values are listed in Supplementary Table 19.
Meta-analysis identifies five new risk genes
Based on results from the first stage, we identified 260 genes with evidence of TDT (TDT statistic39 ≥ 1) and in gene sets enriched with rare inherited LoFs (top 10% LOEUF or within top 20% LOEUF and A-risk ≥ 0.4) (Supplementary Table 6) and 159 genes with P < 0.001 from the DeNovoWEST analysis of DNVs (with 15 genes by both) (Supplementary Table 3). We performed a meta-analysis on the 367 autosomal genes with all data from stage 1 and stage 2, which includes 6,174 new ASD trios, 1,942 new duos, 15,780 unrelated cases (see Methods) and 236,000 population controls.
We used Fisher’s method40 to combine three P values that estimate independent evidence of DNVs, TDT and case-control comparison: (1) DeNovoWEST with DNVs from both stage 1 and stage 2 (n = 23,039 trios, Supplementary Tables 1 and 7) using the parameters estimated in stage 1, (2) TDT with rare LoF variants in parents without ASD diagnoses or intellectual disability with affected offspring in 15,586 trios and 4,907 duos (Supplementary Table 4) and (3) unrelated cases (Supplementary Table 8) compared with population controls using a binomial test. We used two sets of controls: gnomAD exome v2.1.1 non-neuro subset (only samples from individuals who were not ascertained for having a neurological condition in a neurological case-control study, n = 104,068) and TOPMed WGS (freeze 8, n = 132,345). We performed a case-control burden test using the two sets separately and input the larger P value for the meta-analysis. This approach avoids sample overlap and helps ensure that significant genes are not dependent on the choice of population reference. Although population reference data were processed by different pipelines, the cumulative allele frequencies (CAFs) of high-confidence LoF variants (see Methods) are similar between internal pseudocontrols (see Methods) and the two population references after applying the same LoF filters (Supplementary Fig. 5). Previous population genetic simulations predict that for genes under moderate to strong selection (selection coefficient > 0.001), deleterious variants are expected to arise within 1,000 generations and population demographic histories do not confound the CAFs of deleterious alleles in these genes41.
For 367 selected autosomal genes, the point estimates of selection coefficient under the mutation–selection balance model42 are all greater than 0.01 (Supplementary Fig. 6). Most high-confidence LoF variants in these genes are ultra-rare (Supplementary Fig. 7), and the CAFs of high-confidence LoF variants in European and non-European population samples are highly correlated (Supplementary Fig. 8). Therefore, we included population samples across all ancestries as controls. The ultra-rare synonymous variant burden is similar between cases and controls across the selected genes (Extended Data Fig. 3). To make use of all genetic data collected, we also included rare variants of unknown inheritance from ASD cases that were analyzed in the first stage. These variants come from cases that are part of unaffected parent–ASD duos; such variants were either inherited from the parent not participating in the study or DNVs. Therefore, these variants are independent of TDT, even though the same cases were included in TDT.
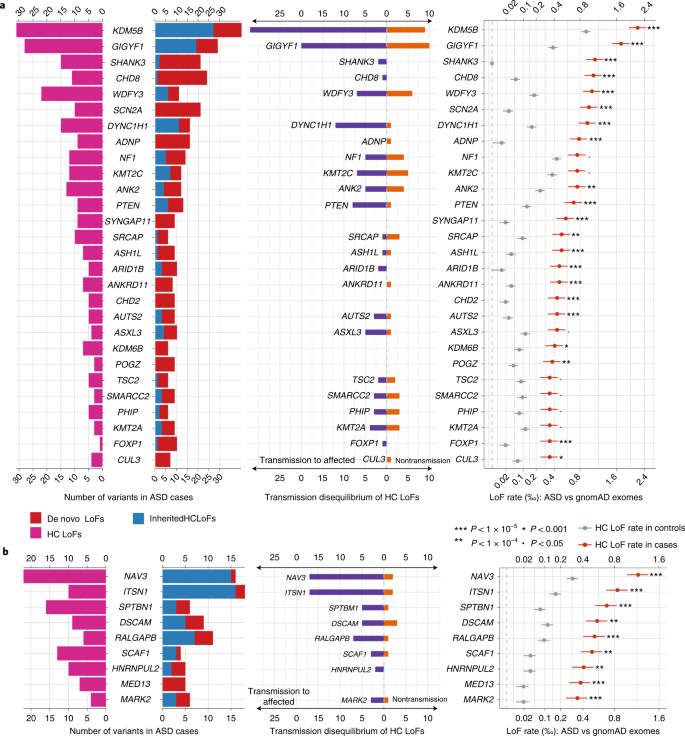
We identified 60 genes with exome-wide significance (P < 2.5 × 10−6), and 72 genes reached study-wide significance accounting for all 5,754 constrained genes (P < 8.7 × 10−6, Supplementary Table 9). Figure 5 summarizes the distribution of LoF variants (with different modes of inheritance) in genes that reached study-wide significance by DNV enrichment (Fig. 5a) and other significant genes by meta-analysis (Fig. 5b and Supplementary Fig. 9). Genes that are significant only in meta-analysis tend to harbor more inherited LoF variants than DNVs, consistent with their lower penetrance for ASD or NDD.
From left to right: pyramid plots summarizing the number of de novo LoFs in 15,857 ASD trios, inherited high-confidence LoFs in 18,720 unrelated offspring included in transmission analysis, and high-confidence LoFs in 15,780 unrelated cases; bar plot of transmission vs nontransmission for rare high-confidence LoFs identified in parents without ASD diagnoses or intellectual disability; three plots comparing the high-confidence LoF rate in 31,976 unrelated ASD cases with gnomAD exomes (non-neuro subset, 104,068 individuals). Horizontal bars are presented as mean values ± standard errors as error bars. a, Twenty-eight known ASD or NDD genes that have LOEUF scores in the top 30% of gnomAD, have a P value for enrichment among all DNVs (P < 9 × 10−6) in 23,039 ASD trios, and have more than 10 LoFs. b, Nine additional ASD risk genes that achieved a P value of <9 × 10−6 in stage 2 of this analysis. The majority of genes in b harbor more inherited LoFs than DNVs. All five novel genes (Table 1) are shown in b. Note that the x axes of LoF rates are in the squared root scale. Poisson test was used to compute the P values. Exact P values are listed in Supplementary Table 6.
Although most significant genes were previously known, we identified five new genes that have exome-wide significance regardless of the choice of population reference: NAV3, MARK2, ITSN1, SCAF1 and HNRNPUL2 (Table 1). The combined P values based on ancestry-specific case-control analyses are similar to the overall case-control analysis for these five genes (Supplementary Table 10). As expected, most supporting variants are ultra-rare, and results are robust to the allele frequency filter. These five new genes together explain 0.27% of the PAR ratio (Supplementary Table 11). NAV3 has a similar PAR to that of CHD8 and SCN2A (~0.095%). ITSN1 is similar to PTEN (~0.065%).
The association of NAV3 with ASD risk is primarily driven by rare inherited variants (Table 1). NAV3 has a high A-risk score, suggesting that the expression pattern of NAV3 is highly similar to known ASD genes (Supplementary Data 1)7,43. NAV3 has high expression in the inner cortical plate of the developing cortex33, and in pyramidal neurons (hippocampus CA1 and somatosensory cortex) and cortical interneurons44,45 (Supplementary Fig. 10). The association of MARK2 with ASD risk is primarily driven by DNVs and is also associated with other NDDs11 (P = 2.7 × 10−5 by DeNovoWEST) including Tourette syndrome46 and epilepsy47. We find that three out of eight autistic offspring with variants in MARK2 report epilepsy, two out of eight report Tourette syndrome and seven out of eight have evidence of cognitive impairment (Supplementary Table 12).
The remaining three novel genes have support from both DNVs and rare LoFs. ITSN1 and SCAF1 show nominal significance of DNV enrichment in 31,058 NDD trios11 (P < 0.05 by DeNovoWEST). SCAF1 was among the top 50 genes from a gene-based burden test in a recent schizophrenia case-control study (P = 0.0027 by burden test)48. Both ITSN1 and NAV3 have moderate effect sizes (point estimate of relative risk 3~6; Supplementary Table 11). ITSN1 has been highlighted in our previous study with evidence of enriched inherited LoFs7. We also assessed deletions in these new genes. For both ITSN1 and NAV3, we identified four partial or whole gene deletions in 33,083 parents without ASD diagnoses or intellectual disability that also show transmission disequilibrium to affected offspring (Extended Data Fig. 4).
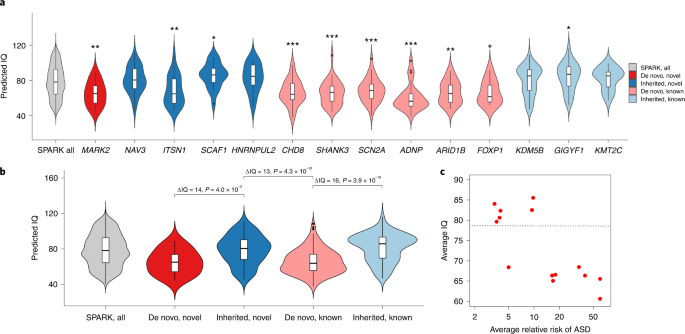
Although both de novo and rare inherited LoFs in the most constrained genes are strongly associated with intellectual disability in ASD (Fig. 4), the association of such variants in individual genes is heterogenous, as suggested by the lack of association of rare inherited variants in genes with high A-risk scores (Extended Data Fig. 2). We calculated the burden of cognitive impairment (see Methods) in 87 ASD individuals with high-confidence LoF variants in the four novel moderate-risk genes and compared it with that in 129 individuals with high-confidence LoF in the well-established ASD risk genes CHD8, SCN2A, SHANK3, ADNP and FOXP1, as well as 8,731 individuals with ASD (Supplementary Fig. 11). Although most individuals with variants in well-established ASD risk genes have some evidence of cognitive impairment (88%), individuals with LoF variants in the moderate-risk genes had significantly lower burden (56%, P = 4.5 × 10−7). Individuals with high-confidence LOFs in the moderate-risk genes did not have a significantly different burden of cognitive impairment than 8,731 individuals with ASD in SPARK (56% vs 50%, P = n.s.). Individuals with LoF variants in the moderate-risk genes also had a similar male:female (4:1) ratio compared with the larger cohort, whereas individuals with variants in the well-established ASD risk genes showed significantly less male bias (1.6:1, P = 0.009) (Supplementary Fig. 11), as previously reported2. We also predicted full-scale intelligence quotient (IQ) on all participants based on parent-reported data using a machine learning method49. Heterozygotes for rare LoFs in three (NAV3, SCAF1 and HNRNPUL2) of the four new genes with substantial contribution from rare inherited variants have similar IQ distribution as the overall SPARK cohort (Fig. 6a), which is substantially higher than heterozygotes with rare LoFs in well-established, highly penetrant genes that contribute to ASD primarily through DNVs (‘DN genes’) such as CHD8, SHANK3 and SCN2A. In fact, both novel and established genes with significant contribution from rare inherited LoFs are less associated with intellectual disability than NDD genes (Fig. 6b). Across these genes, there is a significant negative correlation (r = 0.78, P = 0.001) of estimated relative risk of rare LoFs with average predicted IQ of the individuals with these variants (Fig. 6c).
We examined the distribution of predicted IQ using a machine learning method49 for 95 individuals with ASD with an LoF mutation in one of the five novel exome-wide-significant genes (MARK2, NAV3, ITSN1, SCAF1 and HNRNPUL2) and nine known ASD genes (CHD8, SHANK3, SCN2A, ADNP, ARID1B, FOXP1, KDM5B, GIGYF1 and KMT2C), compared with 2,545 SPARK participants with ASD and known IQ scores. The nine known ASD genes include six genes (pink and labeled ‘de novo, known’) that are well-established de novo ASD risk genes that exceed exome-wide significance and were most frequently identified in SPARK, which maximizes the number of samples available for genotype–phenotype analyses. We also included three genes (light blue and labeled ‘inherited, known’) that have some previous evidence for inherited ASD risk (GIGYF17, KDM5B62 and KMT2C63) and were also frequently identified in SPARK. We denote the genes contributing to ASD primarily through de novo LoF variants in our analysis as ‘de novo’ (red), and the genes primarily through inherited LoF variants as ‘inherited’ (blue). a, Distribution of predicted IQ between individuals with ASD with LoF mutations in the five novel genes, nine known genes and all participants with ASD and known IQ scores in SPARK (n = 2,545). We compared the mean predicted IQ between participants with LoF mutations in ASD genes and all participants by two-sample t-test. *, 0.01 ≤ P < 0.05; **, 0.001 ≤ P < 0.01; ***, P < 0.001. Exact P values are listed in Supplementary Table 19. The box plots represent median as center, and interquartile range (IQR) as bounds of the box; the upper whisker extends from the upper bound of the box to 1.5 × IQR, and the lower whisker extends from the lower bound of the box to 1.5 × IQR. Two-sided t-test was used to compute the P values for comparing mean predicted IQ between ASD individuals with LoF mutation in specific gene and all ASD participants. Individuals with pathogenic variants in de novo risk genes have significantly lower predicted IQ than overall SPARK participants with ASD and known IQ scores, whereas individuals with LoF variants in moderate-risk, inherited genes show similar predicted IQ as the overall SPARK participants, with the exception of ITSN1. b, Distribution of predicted IQ between individuals with ASD gene grouped by both inheritance status (‘de novo’ or ‘inherited’) and whether the ASD genes are novel (‘novel’ or ‘known’). We compared the mean predicted IQ between individuals with pathogenic variants in de novo genes and inherited genes among our five novel genes and nine known genes. Overall, people with LoF mutations in de novo genes have an average of 13–16 points lower predicted IQ than individuals with LoF mutations in inherited genes, regardless of whether the ASD genes are novel or known. The box plots represent median as center, and IQR as the bounds of the box; the upper whisker extends from the upper bound of the box to 1.5 × IQR, and the lower whisker extends from the lower bound of the box to 1.5 × IQR. c, Average relative risk of ASD and average predicted IQ among different groups. Each dot shows the average of individuals with rare LoFs of a gene selected in a. The relative risk is estimated from mega-analysis and capped at 60. Pearson correlation between average IQ and log relative risk is −0.78 (P = 0.001). The horizontal line represents the average IQ (IQ = 79) of all SPARK individuals with predicted IQs. ITSN1 is an outlier at the bottom left corner.
Most known ASD or NDD genes that are enriched by LoF DNVs harbor more de novo than LoF inherited variants in ~16,000 unrelated ASD trios (Fig. 5a and Supplementary Fig. 16), consistent with their high penetrance for ASD or NDD phenotypes and strong negative selection. Using population exome or WGS data, we calculated a point estimate of selection coefficient (\(\hat s\))50 of LoFs in each gene (Supplementary Table 11) and found that the fraction of de novo LoFs in ASD genes is higher in genes with large \(\hat s\), and smaller in genes with small \(\hat s\) (Supplementary Fig. 5b), consistent with population genetic theory51. We also estimated average effect size of rare LoFs in ASD genes by comparing CAF in 31,976 unrelated cases and population exome or WGS data. As expected, known and newly significant ASD genes with higher risk for ASD are under stronger selection (larger \(\hat s\)) (Supplementary Fig. 13).
Functional similarity of new genes and known ASD genes
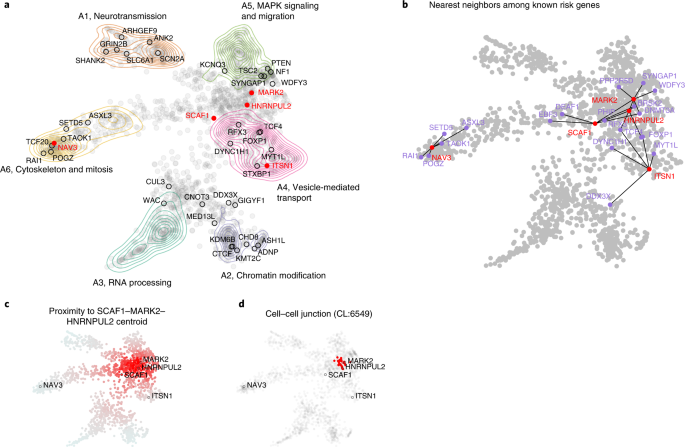
To better appreciate the probable functional implications of the new exome-wide-significant genes that confer inherited risk for ASD, we integrated mechanistic (STRING52) and phenotypic (Human Phenotype Ontology (HPO)53) data into a single embedding space (six dimensions, one for each archetype coefficient) using a combination of canonical correlation analysis and archetypal analysis (see Methods). This embedding space serves as an interpretive framework for putative ASD risk genes (n = 1,776). Six functional or phenotypic archetypes were identified (Fig. 7 and Supplementary Tables 13–15) that represent pathways that are well understood to play a role in ASD: neurotransmission (archetype 1 or A1), chromatin modification (archetype 2 or A2), RNA processing (archetype 3 or A3), vesicle-mediated transport (archetype 4 or A4), MAPK signaling and migration (archetype 5 or A5), and cytoskeleton and mitosis (archetype 6 or A6), also enriched for intermediate filaments. These archetypes organize risk genes in a way that jointly maximizes their association with mechanisms (STRING clusters) and phenotypes (HPO terms). For instance, A1 genes (neurotransmission) are enriched for the STRING cluster CL:8435 (ion channel and neuronal system) and are also associated with seizure and epileptic phenotypes. A2 genes (chromatin modifiers) are enriched for nuclear factors and genes linked to growth and morphological phenotypes (Supplementary Table 14). We call genes that strongly map to an archetype (that is, >2× the next highest-ranking archetype) ‘archetypal’; if this criterion is not met, we call the genes ‘mixed’. Archetypal genes are generally less functionally ambiguous than mixed genes. Of the five novel inherited risk genes, two are archetypal (suggesting function within known risk mechanisms): NAV3 (A6, cytoskeleton and mitosis) and ITSN1 (A4, vesicle-mediated transport). SCAF1, MARK2 and HNRNPUL2 are mixtures of the identified archetypes, largely A4 and A5. That these new genes did not resolve clearly into archetypes (that were defined by known and suspected autism risk genes) suggests that they may operate in potentially novel mechanisms. To elucidate these possibilities, we constructed an ad hoc archetype, defined by the centroid between SCAF1, MARK2 and HNRNPUL2 (see Fig. 7c). Cell–cell junction (CL:6549) was the STRING cluster most associated with this centroid (P = 4.12 × 10−14 by the Kolmogorov–Smirnov test; Fig. 7d), which fits with its location between A4 (vesicle-mediated transport) and A5 (MAPK signaling and migration).
a, Using a combination of archetypal analysis and canonical correlation analysis, putative autism risk genes were organized into k = 6 archetypes that represent distinct mechanistic (STRING) and phenotypic (HPO) categorizations (neurotransmission, chromatin modification, RNA processing, vesicle-mediated transport, MAPK signaling and migration, and cytoskeleton and mitosis). Genes implicated by our meta-analysis are indicated by their label, with novel genes in red. b, For each of the five novel genes, we identified the five nearest neighbors in the embedding space among the 62 meta-analysis genes. SCAF1, MARK2 and HNRNPUL2 were identified as ‘mixed’ rather than ‘archetypal’ in their probable risk mechanisms. c, To gain further insight into possible risk mechanisms, we calculated the embedding distance to the centroid of these three genes, which was then used as an index variable to perform gene set enrichment analysis. d, A STRING cluster (CL:6549) containing genes related to cell–cell junctions and the gap junction was identified as being highly localized in this region of the embedding space (P = 4.12 × 10−14 by the Kolmogorov–Smirnov test). This may suggest that these genes confer autism risk through dysregulation of processes related to cell adhesion and migration.
Power analysis
The power of identifying risk genes with rare inherited variants or DNVs monotonically increases with increasing effect size or expected CAF under the null. New ASD genes to be discovered are likely to have smaller effect size than known ASD genes, as suggested by our results. Additionally, known ASD genes are biased toward longer genes, which have a higher background mutation rate of damaging variants (‘long genes’) (Extended Data Fig. 5). Even though longer genes are more likely to be expressed in the brain and be relevant to ASD or NDD54, among most constrained genes, long genes (LoF mutation rate55,56 above 80% quantile) and short genes (below 80%) have similar enrichment of damaging DNVs and rare inherited LoFs (Supplementary Fig. 14). Notably, for short genes, known genes have virtually no contribution to overtransmitted high-confidence LoFs to affected offspring (Supplementary Fig. 14b), suggesting that many short ASD risk genes remain to be identified.
We used a published framework41 to analyze power based on case-control association of rare variants. For rare variants in genes under strong selection, CAF is largely determined by mutation rate and selection coefficient41. We therefore modeled power of discovering risk genes as a function of relative risk and selection coefficient. With about 5,500 constrained genes, the power of the current study was calculated for 31,976 unrelated cases and an experiment-wise error rate of 9 × 10−6 (Extended Data Fig. 6).
We inversed the power calculation to determine the required sample size to achieve 90% power under the same assumptions (Extended Data Fig. 7). For genes at median LoF mutation rate across all genes, we estimated that it requires about 96,000 cases (three times the current sample size) to identify genes with similar effect size as NAV3 (relative risk = 4.5) and ITSN1 (relative risk = 5), and about 64,000 cases (twice the current sample size) to find genes with similar effect sizes as SCAF1 (relative risk = 8) and HNRNPUL2 (relative risk = 9). We note that ten and five times the current sample size, respectively, are required to detect genes similar to NAV3 or ITSN1 and genes similar to SCAF1 or HNRNPUL2 by DNVs alone.
Discussion
In this study, we identified five new ASD risk genes by both DNVs and rare inherited coding variants. We identified rare LoF variants in new ASD risk genes with modest effect size that are not strongly associated with intellectual disability. This finding represents a difference in phenotypic association with intellectual disability compared with other highly penetrant ASD genes. To find new risk genes with relative risks of 2–5 (comparable to the low relative risk genes from this study, NAV3 and ITSN1) in the 50th percentile for gene-wide LoF mutation rate (2 × 10−6) and the 50th percentile for selection among known risk genes (0.2), our power analysis suggests that 52,000, 73,000, 116,000 or 227,000 total ASD cases are necessary, respectively (cf. equation (1) from power calculation in Supplementary material).
Our results suggest that the identification of new risk genes with rare inherited variants may substantially improve genetic diagnostic yield. We found that rare inherited LoF variants account for 6% of PAR, similar to de novo LoF variants. Over two-thirds of the PAR from coding DNVs is explained by known ASD or NDD genes. In contrast, less than 20% of PAR from rare inherited LoFs variants is explained by known genes, suggesting that most genes contributing to ASD risk through rare inherited variants are yet to be discovered. These unknown risk genes are still largely constrained to LoFs in the general population and/or have similar expression profiles in developing brains to known ASD risk genes. Combining evidence from both DNVs and rare inherited variants, we identified 60 genes associated with ASD with exome-wide significance, including five novel genes. Rare LoFs in these five new genes account for a PAR of 0.27%, about half of the PAR of the five most common highly penetrant ASD genes (KDM5B, GIGYF1, CHD8, SCN2A and SHANK3).
NAV3’s association to autism is primarily driven by rare inherited variants. Carriers of rare LoFs in NAV3 have an average predicted IQ of 81, slightly above the SPARK cohort average (IQ, 79). The prevalence of intellectual disability among NAV3 heterozygotes is similar to the SPARK cohort average. This is distinctly different from established ASD risk genes (for example, CHD8, SHANK3 and SCN2A), nearly all identified by highly penetrant DNVs, and are associated with intellectual disability in ASD cohorts2. The absence of intellectual disability is also observed in other genes (for example, SCAF1, HNRNPUL2, GIGYF1, KDM5B and KMT2C) with substantial contribution from rare inherited variants and modest effect size. Nevertheless, the data show that many individuals with variants in these new ASD genes are affected with various neuropsychiatric conditions such as epilepsy, schizophrenia, Tourette syndrome and attention deficit hyperactivity disorder (ADHD) (Supplementary Table 12). Detailed phenotyping of larger numbers of individuals carrying these rare inherited variants is needed to understand the phenotype associated with each gene. Such strategies should include a genetic and phenotypic assessment of family members who also carry the rare variant without ASD. Because all individuals consented in SPARK are recontactable, such studies will enable a more complete picture of the broad phenotypic effects of these variants without the bias of clinical ascertainment. Overall, these risk genes with modest effect size may represent a different class of ASD genes that are more directly associated with core symptoms of ASD and/or neuropsychiatric conditions rather than global brain development and intellectual disability.
The approaches used in this study made full use of rare variation, and this analytical method is generalizable to many conditions. In particular, the multiple methods used to reduce noise in LoF alleles present in control samples were particularly effective in assessing the signal within the novel genes of moderate effect. We also leveraged gene expression profiles informed by machine learning methods to help prioritize genes for the meta-analysis stage of our analysis38. Future studies that leverage additional multiomic data such as the Genotype–Tissue Expression (GTEx) project may further improve the signal-to-noise ratio.
Our archetypal analysis (Supplementary Tables 13–15) provides some clues to the potential mechanisms of the five newly identified risk genes. ITSN1 was unambiguously mapped to A4 (vesicle-mediated transport) and has a role in coordinating endocytic membrane traffic with the actin cytoskeleton57,58. NAV3 (A6, cytoskeleton and mitosis) is associated with both axon guidance59 and malignant growth and invasion60 and is thought to regulate cytoskeletal dynamics. Indeed, A6 is enriched for processes related to intermediate filaments (Supplementary Table 14), a known determinant of cell motility and polarity61. Although MARK2, SCAF1 and HNRNPUL2 were not identified as archetypal (potentially suggesting divergence from well-known autism risk mechanisms), a search for functional enrichment of this interstitial region between A4 and A5 found that their roles in developmental risk may be most relevant at the cell–cell junction, particularly as it relates to migration (see Fig. 7d).
Taken together, our results suggest that a continued focus on DNVs for ASD gene discovery may yield diminishing returns. By contrast, studies designed to identify genomic risk from rare and common inherited variants will not only yield new mechanistic insight, but also help explain the high heritability of ASD. SPARK is designed to recruit individuals across the autism spectrum, without relying on ascertainment at medical centers. As a result, SPARK may be better suited to identify genes with transmitted variants that have lower penetrance and to identify the genetic contributions to the full spectrum of autism. The strategy used by SPARK—to recruit and assess large numbers of individuals with autism across the spectrum and their available family members without costly, in-depth clinical phenotyping—is necessary to achieve the required sample size to fully elucidate genetic contributions to ASD. The ability to recontact and follow all SPARK participants will also be critical to deeply assess the phenotypes associated with the newly discovered genes and to develop and test novel treatments.
Methods
We established the SPARK cohort to facilitate genotype-driven research of ASD at scale23. All participants were recruited to SPARK under a centralized institutional review board (IRB) protocol (Western IRB Protocol no. 20151664). All participants provided written informed consent to take part in the study. Written informed consent was obtained from all legal guardians or parents for all participants aged 18 and younger and for all participants aged 18 and older who have a legal guardian. Assent was also obtained from dependent participants aged 10 and older. Participants with autism were compensated US$25–50 depending on other registered family members. The mean age of the SPARK cohort in this analysis was 16.5 years (s.d., 19.2 years). Cases made up 46% of the SPARK cohort (the remaining 54% were controls). The sex breakdown of the full SPARK cohort was 57% male and 43% female. The sex breakdown of the SPARK case cohort was 77% male and 23% female.
The first stage of analysis included 28,649 SPARK participants, including 10,242 ASD cases from over 9,000 families with exome sequencing data that passed quality control (Supplementary Data 1). A subset of 1,379 individuals was part of the published pilot study7. To replicate prioritized genes from the discovery stage, we performed a second-stage analysis that included an additional 39,926 individuals with 16,970 ASD cases from over 20,000 families with exome or WGS data available after the analysis in the discovery cohort was completed. New exome sequencing samples in this study were captured using an IDT xGen research panel and sequenced on Illumina NovaSeq. DNA samples were also genotyped for over 600,000 single nucleotide polymorphisms (SNPs) using Infinium Global Screening Array. Supplementary Table 16 outlines the software version and parameter settings for each analysis below. Details on data preprocessing, variant-level quality control procedures, variant annotation, high-confidence LoF variant filtering, and copy number variants, as well as descriptions of other publicly available ASD cohorts (SSC, MSSNG and ASC), are in the Supplementary Note.
DNVs
We identified candidate de novo single nucleotide variants (SNVs) or insertion–deletion mutations (indels) from SPARK and SSC cohorts from per-family variant call formats files (VCFs) generated by GATK64 (v.4.1.2.0) and freebayes65 (v.1.1.0) and a cohort-wide population VCF generated by weCall66 (v.2.0.0) using a set of heuristic filters that aim to maximize the sensitivity while minimizing false negatives in parents7 (Supplementary Table 16). Candidates were retained if they were called by DeepVariant67 (v.0.8.0) in offspring and had no support in parents, identified in multiple offspring that passed the DeepVariant filter in all trios, or were shared by siblings in the same family and the de novo quality estimated by triodenovo was higher than 8 (or 7 for SNVs in CpG context). Before creating the final call set, we selected subsets of variants (see Supplementary Table 16) for manual evaluation by Integrative Genomics Viewer (IGV) to filter out candidates with failed review. Finally, we merged nearby clustered de novo coding variants (within 2 bp for SNVs or 50 bp for indels) on the same haplotype to form multinucleotide variants (MNVs) or complex indels. We removed variants located in regions known to be difficult for variant calling (HLA, mucin, and olfactory receptors). DNVs in the final call set follow a Poisson distribution with an average of 1.4 coding DNVs per affected offspring and 1.3 per unaffected offspring (Supplementary Fig. 15). The proportion of different types of DNVs, the mutation spectrum of SNVs, and indel length distributions were similar between SPARK and SSC (Supplementary Fig. 15). A small fraction of variants in the final call set are likely postzygotic mosaic mutations (Supplementary Fig. 16).
Rare variants
Rare variant genotypes were filtered from cohort-wide population VCFs with quality-control metrics collected from individual and family VCFs (Supplementary Fig. 17a). In brief, we initially extracted high-quality genotypes for each individual for variants that appear in less than 1% of families in the cohort. Evidence for the variant genotypes was re-evaluated by DeepVariant from aligned reads and collapsed over individuals to create site-level summary statistics including the fraction of individual genotypes that passed DeepVariant filter and mean genotype quality over all individuals. For variant genotypes extracted from GLnexus68 (v1.1.3) VCFs, we re-examined variant genotype from per-family VCFs by GATK to collect GATK site-level metrics (including QD, MQ and SOR), then took the read-depth weighted average over families to create cohort-wide site metrics. For variant genotypes extracted from GATK joint genotyping VCFs, these site metrics were directly available from INFO fields.
De novo analysis
In the discovery-stage analysis, the DNV call sets of SPARK and SSC were merged with published DNVs from ASC3,8 and MSSNG6. To infer likely sample overlaps with published trios for which we do not have individual-level data, we tallied the proportion of shared DNVs between all pairs of trios. For a pair of trios, let N1 and N2 be the number of coding DNVs, and let O be the number of shared DNVs between the pair. To account for mutation hot spots, if a DNV is an SNV within CpG context or a known recurrent DNV identified in SPARK and SSC, it contributes 0.5 to the count. Likely overlapping samples were identified if (O/N1) ≥ 0.5 or (O/N2) ≥ 0.5 and they have identical sex.
To determine the expected number of DNVs in the cohort, we used a 7-mer mutation rate model55 in which the expected haploid mutation rate of each base pair depends on the 3-bp sequence context on both sides. The per-base mutation rates were adjusted by the fraction of callable trios at each base pair, which was the fraction of trios with ≥10× coverage in parents and ≥15× coverage in offspring. For published trios, we used an in-house WGS dataset of 300 trios (average, 36× coverage) to approximate the callable regions. Gene-level haploid mutation rates for different classes of DNVs were calculated by summing up the depth-adjusted per-base mutation rate of all possible SNVs of the same class. The rate for frameshift variants was presumed to be 1.3× the rate of stop-gained SNVs56. Mutation rates in haploid X chromosome regions were adjusted for the observed male:female ratio (4.2), assuming that mutation rates in spermatogenesis are 3.4 times higher than in oogenesis9. The exome-wide rate of synonymous DNVs closely matches the observed number of DNVs (Extended Data Fig. 1). We also observed similar fold enrichment of damaging DNVs (vs expected rate) in ASD cases across four cohorts after accounting for samples with family history (Extended Data Fig. 1).
To identify risk genes through DNVs, we applied DeNovoWEST11. We used the empirical burden of DNVs to derive weights for different variant classes in constrained genes (ExAC pLI ≥ 0.5) and nonconstrained genes separately based on positive predictive values (PPVs) (Supplementary Table 18). For ASD, we defined de novo D-mis variants by REVEL score ≥ 0.5. For other NDDs, we defined two classes of de novo D-mis variants by MPC score ≥ 2 or MPC ≤ 2 and CADD score ≥ 25. We first ran DeNovoWEST to test the enrichment of all nonsynonymous DNVs (pEnrichAll). To account for risk genes that harbor only missense variants, we ran DenovoWEST to test the enrichment of de novo missense variants only and applied a second test for spatial clustering of missense variants using denovonear9, then combined evidence of missense enrichment and clustering (pCombMis). The minimal of pEnrichAll and pCombMis was used as the final P value for DeNovoWEST. The exome-wide significance threshold was set to 1.3 × 10−6 (=0.05/(18,000 genes × 2)). The analysis on the replication cohort used the same weights as derived from the discovery cohort. Compared with the original publication11, our implementation of DeNovoWEST used different ways to stratify genes, determine variant weights and calculate per-base mutation rates. We applied our DeNovoWEST implementation on 31,058 NDD trios. The results are highly concordant with published results on the same data (Supplementary Fig. 18). We used P values from our reanalysis on other NDD trios in comparative analysis with ASD.
Gene set enrichment analysis of DNVs was performed using the DNENRICH framework32. We included all de novo LoF and D-mis variants in 5,754 constrained genes from 16,877 ASD and 5,764 control trios. For each gene set, we calculated the fraction of weighted sums of damaging DNVs using PPV weights of constrained genes (Supplementary Table 18) for cases and controls respectively. The test statistic for each gene set is the ratio of such fractions in cases over controls. To determine the distribution of the test statistic under the null hypothesis, we randomly placed mutations onto the exome of all constrained genes, while holding the number of mutations, their trinucleotide context and functional impact to be the same as observed in cases and controls separately. Note that by conditioning on the observed number of damaging DNVs in cases and controls, we tested enriched gene sets in cases that are not due to an increased overall burden. At each round of simulation, the permuted test statistic in each gene set was calculated. Finally, the P value was calculated as the number of times that the permuted statistic was no less than the observed statistic. Fold enrichment was calculated as the ratio between observed and average test statistics over all permutations. We also approximated the 95% confidence interval for fold enrichment by assuming that log[fold enrichment] follows normal distribution with mean 0 and s.d. determined by the P value.
In all DNV analyses above, DNVs shared by full or twin siblings represent single mutational events and were counted only once. When an individual carries multiple DNVs within 100 bp in the same gene, only one variant with the most severe effects was included in the analysis.
Transmission disequilibrium analysis
The effect of inherited LoF variants was analyzed using TDT in each individual gene or in gene sets. Rare LoF variants were first identified in parents without ASD diagnoses or intellectual disability, then for each parent–offspring pair, the number of times the LoF variant was transmitted from parents to offspring was tallied. For variants in the (non-PAR part of the) X chromosome, we only used rare LoF variants carried by mothers without ASD diagnoses or intellectual disability and analyzed transmission in different types of mother–offspring pairs. For TDT analysis of rare inherited missense variants, different D-mis definitions and allele frequency cutoffs were used (Supplementary Fig. 2).
The overtransmission of LoFs to affected offspring was evaluated using a binomial test assuming that under the null hypothesis the chance of transmission is 0.5. In the discovery stage, ultra-rare LoFs with pExt ≥ 0.1 were used in exome-wide transmission disequilibrium and gene set enrichment analysis. For the per-gene test, all rare LoFs with pExt ≥ 0.1 were also used, and the TDT statistic39 for each gene was calculated by \(z = \frac{{T - NT}}{{\sqrt {T + NT} }}\), where T(NT) is the number of times LoF variants were transmitted (not transmitted) to affected offspring. When offspring included monozygotic twin pairs, only one was kept in the transmission analysis. We prioritized 244 autosomal genes with z > 1 in the top 10% of LOEUF or in the top 20% of LOEUF and A-risk ≥ 0.4. In the second stage gene-based test, if a gene-specific pExt threshold was available, we used high-confidence LoF variants that passed the gene-specific pExt filter (see Supplementary Note).
In the gene set enrichment analysis of inherited LoFs, the rate of transmission to affected offspring in each gene set was compared with the transmission rate in the rest of the genes in the background using the χ2 test.
Case-control analysis
Pseudocontrols are constructed from parents without ASD diagnoses or intellectual disability in simplex families, using alleles that were not transmitted to affected offspring. Each parent without ASD diagnoses or intellectual disability contributes a sample size of 0.5 to pseudocontrols. Rare LoFs in ASD cases whose parent data are not available and from other cases that were not used in DNV enrichment or TDT analysis were analyzed in this stage. Specifically, for each ASD case, we found out all of their most recent unaffected ancestors without ASD diagnoses or intellectual disability in the pedigree and calculated the contributing sample size as 1 minus the summation of kinship coefficients with these ancestors. If the contributing sample size was greater than 0, then the sample was included in pseudocases after removing alleles that were observed in any unaffected ancestors without ASD diagnoses or intellectual disability used in TDT and alleles included in DNV analysis if any. Examples of such rare LoFs in cases and their contributing sample sizes are given in Supplementary Fig. 19.
Rare LoFs in cases and controls for the X chromosome were categorized separately for males and females. Male controls include all fathers. In contrast, male cases include only those whose mothers do not have ASD diagnoses or intellectual disability (thus not included in TDT analysis). For females, because we include only mothers without ASD diagnoses or intellectual disability and affected sons in TDT, female pseudocases include all affected females. Female pseudocontrols were established from unaffected mothers in simplex families using alleles that do not transmit to affected sons. Each unaffected mother contributes a sample size of 0.5 to pseudocontrols. In both sexes, DNVs were removed from pseudocases.
For gene-based case-control association in stage 2, we used population data as controls, including gnomAD exomes26 (v2.1.1 non-neuro subset) and TOPMed genomes69 (freeze 8). Variants in the population controls were filtered to keep those that passed the default quality-control filter in released data. For variants in gnomAD data, we further removed variants located in low-complexity regions70 using the same procedure that was already applied to cases and TOPMed data. In gene-level case-control comparison of LoF burden, we used a baseline pExt ≥ 0.1 filter or gene-specific pExt threshold if available to define high-confidence LoF variants (see Supplementary Note). For LoF variants in selected genes, we also extracted curation results by gnomAD to remove curated non-LoF variants, and manually reviewed IGV snapshots from the gnomAD browser if available to remove likely variant calling artifacts (Supplementary Data 1). The number of high-confidence LoF variants was obtained from the allele counts in site-level VCF files. The gene-level burden of high-confidence LoF variants between cases and population controls is tested by comparing the high-confidence LoF variant rates between cases and controls using the Poisson test. The gene-level burden of ultra-rare synonymous variants (allele frequency < 1 × 10−5) between cases and population controls is assessed by a quantile–quantile (Q–Q) plot among all ancestry and European ancestry (Extended Data Fig. 3). To account for difference in depth of coverage, sample sizes are multiplied by the fraction of callable coding regions of each gene (≥15× for autosomes or female X chromosome, ≥10× for male X chromosome) in ASD cases and in population controls respectively.
To account for sample relatedness in case-control analysis, we created a relationship graph in which each node represents an individual and each edge represents a known first-degree or second-degree relationship between two individuals. We also added edges to pairs of individuals without known familial relationship but have an estimated kinship coefficient ≥ 0.1. From the graph, we select one individual from each connected component to create unrelated case-control samples. For the X chromosome, father and sons were treated as unrelated. For population controls, only gnomAD data included sex-specific allele counts and were used in the sex-specific analysis.
Meta-analysis was performed for prioritized autosomal genes that are constrained (defined as ExAC pLI ≥ 0.5 or ranked in the top 20% of the LOEUF scores; Supplementary Data 2). We integrated evidence from the enrichment of all DNVs, transmission disequilibrium and increased burden in cases compared with population controls by combining P values using Fisher’s method40. Study-wide significance was set at 9 × 10−6 (Bonferroni correction with 5,754 constrained genes). In mega-analysis, we combined all unrelated ASD cases together and compared CAFs of high-confidence LoF variants with two population control sets.
Power calculation
To calculate statistical power of the current study and to estimate sample size for future studies, we adopted the statistical framework from ref. 41, comparing CAF of LoF variants in N unrelated cases fcase with CAF f in the general population. The effects of LoFs in the same gene are assumed to be the same (relative risk = γ). We focus only on constrained genes. We assume f to be at mutation–selection equilibrium f = μLoF/s, where μLoF is the LoF mutation rate and s is the selection coefficient. The test statistic asymptotically follows a noncentral χ2 distribution with 1 d.f. and noncentrality parameter (NCP):
Given the significance threshold α, power can be calculated analytically by
where F(x, λ) is the cumulative distribution of \(\chi _1^2\) with NCP λ.
To calculate the sample size to achieve desired power 1 − β at significance level α, we first solve NCP λα,β from the above equation, then estimate sample size by
The current study has a sample size of n = 31,976 and the type I error rate is α = 9 × 10−6 (experimental wide significant). Given continuing expansion of biobank-scale sequencing, treating f as known without error is a reasonable assumption for future studies. To calculate power for the new genes identified in this study, we used point estimates of γ and f from mega-analysis using gnomAD exomes as population controls, and used μLoF computed from the 7-mer context-dependent mutation rate model55 to convert f to s = μLoF/f. The required sample sizes were calculated to achieve 90% power.
Power and sample size are both calculated as a function of relative risk for ASD (γ) and selection coefficient (s) across different haploid LoF mutation rates (μLoF). We only considered s between 0.01 and 0.5, because most prioritized genes have point estimates of s > 0.01 (Supplementary Fig. 5) and genes with s > 0.5 are expected to harbor more de novo than inherited LoF variants and can be identified from the enrichment of DNVs. We limited relative risk (γ) between 1 and 20 to focus on risk genes with moderate to small effects. The reduction in fitness s is correlated with the increases in ASD risk γ by s = γπ under the assumption of no pleiotropic effect, where π is ASD prevalence and sD is decreased reproductive fitness of ASD cases. Based on epidemiological studies, the current estimated prevalence of ASD is \(\hat \pi = 1/54\)71, and the estimated sD is for 0.75 male and for 0.52 female72, so sex-averaged \(\hat s_D = 0.71\) (assuming male:female ratio of 4.2). In reality, most known ASD genes also show pleiotropic effects with other NDDs or are associated with prenatal death; therefore, \(s \ge \gamma \pi s_D \approx \gamma \hat \pi \hat s_D = 0.013\gamma\). So, we considered only combinations of (s, γ) that satisfy the condition s ≥ 0.013γ.
Gene sets
To evaluate the contribution of known ASD risk genes to the burdens of DNVs and inherited LoF variants identified in this study, we collected 618 known dominant ASD or NDD genes from the following sources: (1) Known NDD genes from DDG2P73 (2020-02) that are dominant or X-linked and have an organ specificity list that includes the brain or cause multisystem syndrome; (2) high-confidence ASD genes collected by the Simons Foundation Autism Research Initiative (SFARI)74 (2019-08) with score of 1 or 2, excluding known recessive genes; and (3) dominant ASD genes reported in recent literature and included in the SPARK genes list75 (2020-07).
To evaluate the gene sets enriched by damaging DNVs or inherited high-confidence LoFs, we used all constrained genes by ExAC pLI ≥ 0.5 or in the top 20% of the LOEUF as the background. Gene sets of the following five categories were collected for gene set enrichment analysis.
Transcriptome and proteome
(1) Genes with brain-specific expression, defined as the genes with average reads per kilobase of transcript per million mapped reads (RPKM) > 1 in the brain and over four times the median RPKM of 27 tissues in processed RNA sequencing (RNA-seq) data from ref. 76. (2) Genes in coexpression modules M2 and M3 derived from weighted gene correlation network analysis (WGCNA) of BrainSpan developmental RNA-seq data, previously reported to be enriched for known ASD genes33. (3) Genes expressed in excitatory or inhibitory neurons. We selected genes from ref. 77 that have average transcripts per million (TPM) > 100 in excitatory neurons and in inhibitory neurons. (4) Synaptic genes collected from SynaptomeDB78.
Neuronal regulome
(1) Putative CELF4 target genes, defined as genes with an iCLIP occupancy > 0.2 in ref. 79. (2) CHD8 target genes, defined as genes with promoter or enhancer regions that overlap with CHD8 binding peaks in human neural stem cells or midfetal brain in ref. 36. (3) FMRP target genes35 with a false discovery rate (FDR) < 0.1 that mapped them to orthologous human genes (Mouse Genome Informatics80, 2018-07). (4) RBFOX2 target genes34 that have Rbfox2 tag counts ≥ 8. Due to high correlations between RBFOX1 and RBFOX3, targeted genes by the two RNA binding proteins were merged in one gene set and selected to have total Rbfox1 and Rbfox3 tag counts > 24.
Autism gene predictions
(1) ForecASD is an ensemble classifier that integrates brain gene expression, heterogeneous network data and previous gene-level predictors of autism association to yield a single prediction score37. We created two sets of genes with forecASD prediction score greater than 0.4 or 0.5. (2) A-risk is a classifier that uses a gradient boosting tree to predict autism candidate genes using cell-type specific expression signatures in the fetal brain38. We created three sets of genes with prediction score greater than 0.4, 0.5 or 0.6.
Genetic evidence
(1) Genes enriched with DNVs in ASD, with nominal statistical evidence (P < 0.01 or P < 0.05 by DeNovoWEST) in the SPARK discovery cohort of 16,877 trios. (2) Genes enriched with DNVs in other NDDs (Supplementary Data 3), with nominal statistical evidence in 31,058 NDDs11. (3) Genes implicated in schizophrenia, with nominal statistical evidence (P < 0.05)48.
Genetic constraint
Four constraint gene sets are defined by genes in top 10% of the gnomAD LOEUF, 10–20% the gnomAD LOEUF and genes with selection coefficient for heterozygous protein-truncating variants estimated from ExAC data (sHet) ≥ 0.25 and ≥ 0.2.
Archetypal analysis
Archetypal analysis81 is an unsupervised learning approach that has similarities to other dimensionality reduction and clustering approaches. An important distinction of archetypal analysis from comparable approaches is that it seeks a set of k archetypes, which are points along the convex hull of the data, from which all data points may be expressed as a mixture. The output of archetypal analysis is an N by k matrix, α, of [0,1] coefficients that represent the contribution of each archetype to each data point. Whereas cluster centroids are embedded within the interior of a cluster, archetypes are, by design, at the extremes of the data (that is, along the convex hull). In practice, this ensures that the archetypes are appreciably distinct from one another, which is often not the case for cluster centroids. Here, we use archetypal analysis as a means to organize putative ASD risk genes in a space that has meaning from a mechanistic (STRING) and phenotypic (HPO) standpoint. Genes of particular interest to this study can then be considered and interpreted in the context of the archetypes that define this space. STRING (v11)52 clusters and HPO53 terms were formatted as gene-by-term binary matrices. The working gene list was taken as the union of forecASD top decile genes and the 62 autism-associated genes from this study (total of 1,776 genes). A total of 583 genes from this set had annotations in both STRING and HPO, and using these genes, a canonical correlation analysis (CCA) was carried out using the RGCCA package for R (https://cran.r-project.org/web/packages/RGCCA/index.html) using five components and sparsity parameter c1 set to 0.8 for both the HPO and STRING matrices. Component scores for all 1,776 genes were calculated using the STRING cluster annotations and the corresponding coefficients from the CCA and was used as input for archetypal analysis81, with the optimal k (number of archetypes) selected using the elbow plot heuristic82 and the residual sums of squares (RSS) plotted as a function of k. We displayed the archetypal embedding using the simplexplot() function of the archetypes R package. Genes were identified as ‘archetypal’ if their top archetype coefficient was >2× the next highest archetypal coefficient. Those genes that did not fulfill this criterion were classified as ‘mixed’, whereas those that did were assigned to their maximally scoring archetype. We applied the following heuristic to guide the naming of each archetype: each MSigDB83 gene set (v7.4) that has two or more autism genes (n = 62 as described above) annotated is tested for association with the archetypal coefficients (A1–A6, simultaneously) in a quasibinomial generalized linear model (GLM) (Supplementary Table 15). The one-sided association P value (that is, positive association) for each archetype is reported in Supplementary Table 12 (top 20 for each archetype). To name each archetype, the top 20 gene sets with the strongest associations with that archetype were considered; this leads to the following naming conventions: A1, neurotransmission; A2, chromatin modification; A3, RNA processing; A4, vesicle-mediated transport; A5, MAPK signaling and migration; and A6, cytoskeleton and mitosis (see Supplementary Table 15 and Fig. 7). Representative genes for each archetype were chosen from among the list of 62 risk genes identified in this study, using the top six genes for each archetype (note that these genes do not necessarily fulfill the ‘archetypal’ criterion described above, but are simply the top six of the 62 for each archetype).
Statistics and reproducibility
No statistical method was used to predetermine sample size, and no data were excluded from the analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
In order to abide by the informed consents that individuals with autism and their family members signed when agreeing to participate in a SFARI cohort (SSC and SPARK), researchers must be approved by SFARI Base (https://base.sfari.org). To access to SPARK or SFARI data, researchers should
(1) Obtain a SFARI Base account at https://base.sfari.org, which will require affiliating with an institution. Currently, there are 271 institutions around the world that have signed SFARI’s Researcher Distribution Agreement (RDA), and any researcher affiliated with those institutions can apply for SFARI Base access.
(2) Review the institute’s executed RDA. The standard RDA is available at https://s3.amazonaws.com/sf-web-assets-prod/wp-content/uploads/sites/2/2021/06/15165956/SFARI_RDA.pdf
(3) Create a SFARI Base project, which includes a title, abstract and an IRB approval or exemption document.
(4) Create a SFARI Base request. All requests are processed in a timely manner.
The SPARK data is accessible as follows:
SFARI_SPARK_iWES includes exome and genotyping data on 70,487 participants, including all people analyzed in this paper plus an additional 11,282 participants.
SFARI_SPARK_WGS_1 includes whole genome data from 2,629 individuals from 645 families with at least one person with autism.
SFARI_SPARK_WGS_2 includes whole genome data from 2,365 individuals from 587 families with at least one person with autism.
SFARI_SPARK_WGS_3 includes whole genome data from 2,871 individuals from 803 families with at least one person with autism.
SSC_WES_3 is whole exome data on the Simons Simplex Collection (SSC) as analyzed and reported in ref. 19.
SFARI_SSC_WGS_pilot contains genomes of 40 families with autism.
SFARI_SSC_WGS_1 and SFARI_SSC_ WGS_2 contain WGS of the SSC.
SSC Dataset contains phenotypic information on 2,644 simplex autism families.
SPARK Phenotype Dataset V7 is the current phenotypic dataset on 290,502 SPARK participants, including 111,720 participants with autism.
Code availability
All software used in this study is publicly available. The code for major figures and analysis can be found at https://github.com/ShenLab/SPARK_Analysis_V1.git or https://doi.org/10.5281/zenodo.6646871.
References
Lord, C. et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 6, 5 (2020).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
O’Roak, B. J. et al. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat. Commun. 5, 5595 (2014).
Yuen, R. K. C. et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat. Med. 21, 185–191 (2015).
Yuen, R. K. C. et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat. Neurosci. 20, 602–611 (2017).
Feliciano, P. et al. Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. npj Genomic Med 4, 19 (2019).
Satterstrom, F. K. et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584.e23 (2020).
The Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature 519, 223–228 (2015).
The Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature 542, 433–438 (2017).
Kaplanis, J. et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature 586, 757–762 (2020).
He, X. et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet. 9, e1003671 (2013).
Nguyen, H. T. et al. Integrated Bayesian analysis of rare exonic variants to identify risk genes for schizophrenia and neurodevelopmental disorders. Genome Med. 9, 114 (2017).
Gaugler, T. et al. Most genetic risk for autism resides with common variation. Nat. Genet. 46, 881–885 (2014).
Sandin, S. et al. The familial risk of autism. JAMA 311, 1770–1777 (2014).
Sandin, S. et al. The heritability of autism spectrum disorder. JAMA 318, 1182–1184 (2017).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Krumm, N. et al. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 47, 582–588 (2015).
Kosmicki, J. A. et al. Refining the role of de novo protein-truncating variants in neurodevelopmental disorders by using population reference samples. Nat. Genet. 49, 504–510 (2017).
Ruzzo, E. K. et al. Inherited and de novo genetic risk for autism impacts shared networks. Cell 178, 850–866.e26 (2019).
Wilfert, A. B. et al. Recent ultra-rare inherited variants implicate new autism candidate risk genes. Nat. Genet. 53, 1125–1134 (2021).
The SPARK Consortium. SPARK: a US cohort of 50,000 families to accelerate autism research. Neuron 97, 488–493 (2018).
MacArthur, D. G. & Tyler-Smith, C. Loss-of-function variants in the genomes of healthy humans. Hum. Mol. Genet. 19, R125–R130 (2010).
MacArthur, D. G. et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science 335, 823–828 (2012).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Cummings, B. B. et al. Transcript expression-aware annotation improves rare variant interpretation. Nature 581, 452–458 (2020).
Cassa, C. A. et al. Estimating the selective effects of heterozygous protein-truncating variants from human exome data. Nat. Genet. 49, 806–810 (2017).
Fuller, Z. L., Berg, J. J., Mostafavi, H., Sella, G. & Przeworski, M. Measuring intolerance to mutation in human genetics. Nat. Genet. 51, 772–776 (2019).
An, J.-Y. et al. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science 362, aat6576 (2018).
Ioannidis, N. M. et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 99, 877–885 (2016).
Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184 (2014).
Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, 1008–1021 (2013).
Weyn-Vanhentenryck, S. M. et al. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep. 6, 1139–1152 (2014).
Darnell, J. C. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011).
Cotney, J. et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 6, 6404 (2015).
Brueggeman, L., Koomar, T. & Michaelson, J. J. Forecasting risk gene discovery in autism with machine learning and genome-scale data. Sci. Rep. 10, 4569 (2020).
Chen, S. et al. Dissecting autism genetic risk using single-cell RNA-seq data. Preprint at https://www.bioRxiv.org/content/10.1101/2020.06.15.153031v1 (2020).
Ewens, W. J. & Spielman, R. S. The transmission/disequilibrium test: history, subdivision, and admixture. Am. J. Hum. Genet. 57, 455–464 (1995).
Fisher, R. A. Statistical Methods for Research Workers 11th edn, rev (Oliver and Boyd, 1925).
Zuk, O. et al. Searching for missing heritability: designing rare variant association studies. Proc. Natl Acad. Sci. USA 111, E455–E464 (2014).
Wright, S. Evolution in Mendelian populations. Genetics 16, 97–159 (1931).
Skene, N. G. & Grant, S. G. N. Identification of vulnerable cell types in major brain disorders using single cell transcriptomes and expression weighted cell type enrichment. Front. Neurosci. 10, 16 (2016).
Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155, 997–1007 (2013).
Chang, J., Gilman, S. R., Chiang, A. H., Sanders, S. J. & Vitkup, D. Genotype to phenotype relationships in autism spectrum disorders. Nat. Neurosci. 18, 191–198 (2015).
Wang, S. et al. De novo sequence and copy number variants are strongly associated with Tourette disorder and implicate cell polarity in pathogenesis. Cell Rep. 24, 3441–3454.e12 (2018).
Heyne, H. O. et al. De novo variants in neurodevelopmental disorders with epilepsy. Nat. Genet. 50, 1048–1053 (2018).
Singh, T. et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516 (2022).
Shu, C., Snyder, L. G., Shen, Y. & Chung, W. K. The SPARK Consortium. Imputing cognitive impairment in SPARK, a large autism cohort. Autism Res. 15, 156–170 (2022).
Arnheim, N. & Calabrese, P. Understanding what determines the frequency and pattern of human germline mutations. Nat. Rev. Genet. 10, 478–488 (2009).
Rees, E., Moskvina, V., Owen, M. J., O’Donovan, M. C. & Kirov, G. De novo rates and selection of schizophrenia-associated copy number variants. Biol. Psychiatry 70, 1109–1114 (2011).
Szklarczyk, D. et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Köhler, S. et al. The Human Phenotype Ontology in 2021. Nucleic Acids Res. 49, D1207–D1217 (2021).
Raychaudhuri, S. et al. Accurately assessing the risk of schizophrenia conferred by rare copy-number variation affecting genes with brain function. PLoS Genet. 6, e1001097 (2010).
Carlson, J. et al. Extremely rare variants reveal patterns of germline mutation rate heterogeneity in humans. Nat. Commun. 9, 3753 (2018).
Samocha, K. E. et al. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 46, 944–950 (2014).
Yamabhai, M. et al. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J. Biol. Chem. 273, 31401–31407 (1998).
Wang, W. et al. ITSN-1 controls vesicle recycling at the neuromuscular junction and functions in parallel with DAB-1. Traffic 9, 742–754 (2008).
Stringham, E. G. & Schmidt, K. L. Navigating the cell: UNC-53 and the navigators, a family of cytoskeletal regulators with multiple roles in cell migration, outgrowth and trafficking. Cell Adhes. Migr. 3, 342–346 (2009).
Karenko, L. et al. Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue. Cancer Res. 65, 8101–8110 (2005).
Sanghvi-Shah, R. & Weber, G. F. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front Cell Dev. Biol. 5, 81 (2017).
Martin, H. C. et al. Quantifying the contribution of recessive coding variation to developmental disorders. Science 362, 1161–1164 (2018).
Guo, H. et al. Inherited and multiple de novo mutations in autism/developmental delay risk genes suggest a multifactorial model. Mol. Autism 9, 64 (2018).
Poplin, R. et al. Scaling accurate genetic variant discovery to tens of thousands of samples. Preprint at https://www.bioRxiv.org/content/10.1101/201178v3 (2018).
Garrison, E. & Marth, G. Haplotype-based variant detection from short-read sequencing. Preprint at https://arXiv.org/abs/1207.3907 (2012).
GENOMICS plc - weCall: fast, accurate and simple to use command line tool for variant detection in NGS data. https://github.com/Genomicsplc/wecall
Poplin, R. et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotechnol. 36, 983–987 (2018).
Lin, M. F. et al. GLnexus: joint variant calling for large cohort sequencing. Preprint at https://www.bioRxiv.org/content/10.1101/343970v1 (2018).
Taliun, D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021).
Li, H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics 30, 2843–2851 (2014).
Maenner, M. J. et al. Prevalence of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 69, 1–12 (2020).
Power, R. A. et al. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry 70, 22–30 (2013).
Wright, C. F. et al. Genetic diagnosis of developmental disorders in the DDD study: a scalable analysis of genome-wide research data. Lancet 385, 1305–1314 (2015).
Abrahams, B. S. et al. SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism 4, 36 (2013).
Singer, E. What makes an autism gene? https://SPARKforAutism.org/discover_article/what-makes-an-autism-gene/ (2017).
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 13, 397–406 (2014).
Mo, A. et al. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron 86, 1369–1384 (2015).
Pirooznia, M. et al. SynaptomeDB: an ontology-based knowledgebase for synaptic genes. Bioinformatics 28, 897–899 (2012).
Wagnon, J. L. et al. CELF4 regulates translation and local abundance of a vast set of mRNAs, including genes associated with regulation of synaptic function. PLoS Genet. 8, e1003067 (2012).
Eppig, J. T. Mouse Genome Informatics (MGI) Resource: genetic, genomic, and biological knowledgebase for the laboratory mouse. ILAR J. 58, 17–41 (2017).
Cutler, A. & Breiman, L. Archetypal analysis. Technometrics 36, 338–347 (1994).
Thorndike, R. L. Who belongs in the family? Psychometrika 18, 267–276 (1953).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. 102, 15545–15550 (2005).
Packer, J. S. et al. CLAMMS: a scalable algorithm for calling common and rare copy number variants from exome sequencing data. Bioinformatics 32, 133–135 (2016).
Acknowledgements
We are extremely grateful to the thousands of individuals and families who are participating in this study. We thank the sites, staff and volunteers of the SPARK Clinical Site Network and SFARI for their invaluable contributions. We are grateful to the many ASD advocacy and service organizations that have helped us inform the community about SPARK, including the Autism Society of America and its affiliates, the Global and Regional Autism Spectrum Partnership (GRASP), Autism Speaks, and the Autism Science Foundation. We thank the members of SPARK’s Community Advisory Council for providing feedback and advice. We thank members of our Scientific Advisory Board and SFARI scientists for advice on our protocol and participant outreach and retention strategies. Our de novo analyses involved primary data from SSC and results from published ASD studies, including ASC and MSSNG, and our case-control analysis used published population controls from gnomAD (exome v2.1.1, non-neuro subset) and TOPMed (WGS, freeze 8). Approved researchers can obtain the SPARK population dataset described in this study by applying at https://base.sfari.org. The SPARK initiative is funded by the Simons Foundation as part of SFARI. This research was supported, in part, by a grant from the National Institute of Mental Health (NIMH R01MH101221) and grants from the Simons Foundation (SFARI nos. 608045 and 810018EE) to E.E.E., a grant from the National Institutes of Health (NIH R01GM120609) and from the Simons Foundation (SFARI no. 606450) to Y.S., a grant from the Simons Foundation (SFARI no. 644038) to B.J.O. and J.J.M., a grant from the National Institute of Mental Health to T.N.T. (NIMH 1K99MH117165), and grants MH105527 and DC014489 from the National Institute of Health to J.J.M. E.E.E. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Consortia
Contributions
I. Astrovskaya, J.B.H., J.J.M., N.V., P.F., C. Shu, T.W., W.K.C., X.Z. and Y.S. designed and conceived this study. A. Adams, A. Andrus, A. Berman, A. Brown, A.C., A.C.G., A.D.S., A.E., A. Fanta, A. Fatemi, A. Fish, A. Goler, A. Gonzalez, A. Gutierrez Jr., A. Hardan, A. Hess, A. Hirshman, A. Holbrook, A.J.A., A.J. Griswold, A. Jarratt, A. Jelinek, A. Jorgenson, A. Juarez, A. Kim, A. Kitaygorodsky, A.L., A.L.R., A.L.W., A.M.D., A. Mankar, A. Mason, A. Miceli, A. Milliken, A.M.-L., A.N.S., A. Nguyen, A. Nicholson, A. Nishida, A.P., A.P.M., A.R.G., A. Raven, A. Rhea, A. Simon, A. Swanson, A. Sziklay, A. Tallbull, A. Tesng, A.W., A.Z., B.A.H., B.B., B.E., B.E.R., B. Hauf, B.J.O., B.L., B.M.V., B.S., B.V., C.A.E., C.A.W.S., C. Albright, C. Anglo, C.B., C.C.B., C.C.-S., C. Cohen, C. Colombi, C.D., C.E., C.E.R., C. Fassler, C. Gray, C. Gunter, C.H.W., C.K., C. Leonczyk, C.L.M., C. Lord, C.M.T., C.M., C.O.-L., C. Ortiz, C.P., C.R.R., C. Roche, C. Shrier, C. Smith, C.V., C.W.-L., C. Zaro, C. Zha, D.B., Daniel Cho, D. Correa, D.E.S., D.G., D.G.A., D.H., D.I., D.L.C., D. Li, D. Limon, D. Limpoco, D.P., D. Rambeck, D. Rojas, D. Srishyla, D. Stamps, E.A.F., E. Bahl, E.B.-K., E. Blank, E. Bower, E. Brooks, E.C., E. Dillon, E. Doyle, E. Given, E. Grimes, E.J., E.J.F., E.K., E.L.W., E. Lamarche, E. Lampert, E.M.B., E. O’Connor, E. Ocampo, E. Orrick, E.P., E.R., E.S., E.T.M., E.V.P., F.F., F.K.M., G.A., G.B., G.D., G.H., G.M., G.S., G.S.D., G.T., H.C., H.E.K., H.G., H.H., H.K., H.L.S., H. Lechniak, H. Li, H.M., H.R., H. Zaydens, I. Arriaga, I.F.T., J.A., J.A.G., J. Beeson, J. Brown, J. Comitre, J. Cordova, J.D., J.F.C., J.F.H., J. Gong, J. Gunderson, J.H., J.J.M., J. Judge, J. Jurayj, J.K.L., J. Manoharan, J. Montezuma, J.N., J.O., J. Pandey, J. Piven, J. Polanco, J. Polite, J.R., J.R.W., J.S., J.S.S., J.T.M., J. Tjernagel, J. Toroney, J.V.-V., J.W., K.A., K.A.S., K. Baalman, K. Beard, K. Callahan, K. Coleman, K.D.F., K. Dent, K. Diehl, K.G., K.G.P., K.H., K.L.P., K. Murillo, K. Murray, K.N., K.O., K. Pama, K.R., K. Singer, K. Smith, K. Stephenson, K.T., L.A., L.A.C., L. Beeson, L. Carpenter, L. Casten, L. Coppola, L. Cordiero, L.D., L.D.P., L.F.C., L.G.S., L.H.S., L.K.W., L.L., L.M.H., L.M.P., L. Malloch, L. Mann, L.P.G., L.S., L.V.S., L.W., L.Y., L.Y.-H., M.A., M. Baer, M. Beckwith, M. Casseus, M. Coughlin, M. Currin, M. Cutri, M. DuBois, M. Dunlevy, M.F., M.F.G., M.G., M. Haley, M. Heyman, M. Hojlo, M.J., M.J.M., M. Kowanda, M. Koza, M.L., M.M., M.N., M.N.H., M.O., M.P., M.R., M. Sabiha, M. Sahin, M. Sarris, M. Shir, M. Siegel, M. Steele, M. Sweeney, M.T., M.V.-M., M. Verdi, M.Y.D., N.A., N. Bardett, N. Berger, N.C., N.D., N.G., N.H., N. Lillie, N. Long, N.M.R.-P., N. Madi, N. Mccoy, N.N., N. Rodriguez, N. Russell, N.S., N. Takahashi, N. Targalia, N.V., O.N., O.Y.O., P.F., P.H., P.M., P.S.C., R.A.B., R.A.G., R.C.S., R.D.A., R.D.C., R.J., R.J.L., R.K.E., R.L., R.P.G.-K., R. Remington, R.S., R.T.S., S.A., S. Birdwell, S. Boland, S. Booker, S. Carpenter, S. Chintalapalli, S. Conyers, S.D., S.D.B., S.E., S.F., S.G., S. Hepburn, S. Horner, S. Hunter, S.J.B., S.J.L., S. Jacob, S. Jean, S. Kim, S. Kramer, S.L.F., S. Licona, S. Littlefield, S.M.K., S. Mastel, S. Mathai, S. Melnyk, S. Michaels, S. Mohiuddin, S. Palmer, S. Plate, S.Q., S.R., S. Sandhu, S. Santangelo, S. Skinner, S.T., S. Xu, S. Xiao, Sabrina White, Stormi White, T.C., T.G., T.H., T.I., T.K., T.P., T.R., T.S., T.R.T., T.T., V. Galbraith, V. Gazestani, V.J.M., V.R., V.S., W.C.W., W. Cal, W.K.C., W.S.Y., Y.C. and Z.E.W. recruited participants and collected clinical data and biospecimens. A. Amatya, A. Bashar, A.E.L., A. Mankar, A. Nguyen, B.J., C. Rigby, Dave Cho, D.V.M., E. O’Connor, J.A., M.D.M., M.E.B., N. Lawson, N. Lo, N.V., R.M., R. Rana, S.G., S. Jean, S. Shah and W. Chin built and supported the SPARKforAutism.org website, software, databases and systems, and managed SPARK data. A.D.K., A.J. Gruber, A. Nishida, B. Han, B.J.O., C. Fleisch, C. Shu, D.V.M., E. Brooks, G.J.F., I. Astrovskaya, J.B.H., J.J.M., J.R.W., J.U.O., L. Brueggeman, L.G.S., M.A.P., N. Lo, N.V., O.M., P.F., S.D.B., S.C.M., S.X.X., T.N.T., T.S.C., T.W., W.T.H., X.Z. and Y.S. performed analyses, processed biospecimens and sequenced DNA samples. A. Fatemi, A. Kitaygorodsky, A. Soucy, C. Shu, D.H.G., E.B.-K., E.E.E., E.R., H.Q., H. Zhang, H. Zhao, I. Astrovskaya, J.B.H., J.J.M., J.R.W., J.U.O., L. Brueggeman, L.G.S., M.Y.D., N.V., O.M., P.F., R.N.D., S.D.B., S.C.M., S.X.X., T.K., T.N.T., T.P., T.S., T.R.T., T.W., T.W.Y., W.K.C., X.Z. and Y.S. helped with data interpretation. A.E.L., E.E.E., J.J.M., N.V., P.F., W.K.C. and X.Z. supervised the work. B.J.O., I. Astrovskaya, J.B.H., J.J.M., J.U.O., C. Shu, J.R.W., N.V., P.F., T.N.T., T.W., W.K.C., X.Z. and Y.S. wrote this paper.
Corresponding author
Ethics declarations
Competing interests
D.H.G. has received consulting fees or equity participation for scientific advisory board work from Ovid Therapeutics, Axial Biotherapeutics, Acurastem, and Falcon Computing. E.E.E. serves on the Scientific Advisory Board of Variant Bio. W.K.C. serves on Scientific Advisory Board of the Regeneron Genetics Center and is the Director of Clinical Research for SFARI. All other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Anders Borglum and Xin He for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overall burden of de novo variants in four ASD cohorts included in the discovery sample.
(A) Observed rates of de novo LoF, Dmis (REVEL > = 0.5) and silent variants (B) are compared with expected rates. We used a 7mer sequence context dependent mutation rate model55 to calculate expected rates for different classes of de novo variants after adjusting sequencing coverage, and found a close match with observed de novo rates in control trios. The rates of de novo LoF and Dmis variants in ASD cases are significantly higher than baseline expectation and are reduced in cases with known family history. Data are presented as mean values and 95% confidence interval as error bars.
Extended Data Fig. 2 Enrichment of de novo damaging and rare, inherited LoF variants in ASD cases across gene sets.
Gene sets were defined and grouped by transcriptome proteome, neuronal regulome, ASD gene prediction scores, genetic evidence from neuropsychiatric diseases, and gene level constraint. Analyses were repeated after removing known ASD/NDD genes. Number of genes in each set before and after removing known genes are shown in bracket below gene set. Dots represent fold enrichment of DNVs or odds ratio for over-transmission of LoFs in each set. Horizontal bars indicate 95% confidence interval. For each gene set, also shown are the percentage of excessive DNVs in cases and percentage of over-transmission of rare, inherited LoFs to cases. (A) De novo enrichment analysis was performed by dnEnrich that conditional on the overall increase in burden of de novo damaging variants in cases compared with controls (Methods). P-values were derived from 100,000 random permutations of de novo damaging variants among all 5,754 constrained genes and accounts for the tri-nucleotide sequence context and gene length. (B) Enrichment of rare, inherited LoFs was evaluated by comparing the transmission and non-transmission of ultra-rare LoFs with pExt > =0.1 in the gene set versus those in all other constrained genes using a 2-by-2 table. P-values were given by chi-squared test.
Extended Data Fig. 3 QQ plot showing the ultra-rare synonymous burden test among 404 selected genes between SPARK cases and gnomAD controls for allele frequency<1e-5.
HMCN2 is excluded (not shown) since it has poor coverage in gnomAD. Panel A shows the cross-ancestry case-control ultra-rare synonymous burden comparison, while Panel B shows the European-only case-control ultra-rare synonymous burden comparison. The observed P values for each gene are sorted from largest to smallest and plotted against expected values from a theoretical chi-square distribution.
Extended Data Fig. 4 Transmission disequilibrium of exonic or single gene deletions of ITSN1 (A) and NAV3 (B).
The read depth signal plots show normalized read depth (NDP) of exome targets used in CNV calling by CLAMMS84 for ITSN1 (A) and NAV3 (B) in Family 1–8. NDPs of −0.5, 0 and 0.5 correspond to copy number (CN) of 1 (deletion), 2 (normal diploid) and 3 (duplication). NDPs of exon targets within deleted regions are colored red. Dark and gray areas correspond to 1 and 2 estimated standard deviations of NDP for each exon target. CN deletions were initially discovered from all unaffected parents, then subsequently genotyped on Family 1–8. Signal plots of Family 1–8 are shown with parents appear in the top and offspring in the bottom. The deletion regions and affected exons of genes are shown below each plot.
Extended Data Fig. 5 Cumulative distribution of haploid mutation rates (per generation) of LoF and D-mis (REVEL > = 0.5) variants of all protein coding genes on autosomes.
Panel A shows the distribution of all autosomal genes vs. known ASD/NDD genes. Panel B shows all autosomal genes vs. prioritized genes. Baseline mutation rates were calculated using 7mer sequence context dependent mutation rates55. Known ASD/NDD genes tend to have higher mutation rates than average genes.
Extended Data Fig. 6 Power of case-control association by rare LoFs variants (‘mega-analysis’) with sample size equal to current study.
The mega-analysis of current study compares the rate of LoF variants in 32,024 unrelated ASD cases with population controls with sample sizes about 76,000~132,000. For power calculation, we assumed that population controls are infinite so that cumulative allele frequency are known and presumed to be at equilibrium under selection-mutation balance for constrained genes (f = μLoF/s). Experiment-wide error rate was set at 9e-6 (0.05 divided by the number of autosomal genes at gnomAD LOEUF 30%). Power is calculated as a function of relative risk for ASD (RR) and selection coefficient (s) across different haploid LoF mutation rates (μLoF) using an analytic approximation by Zuk et al.41. We only considered selection coefficient between 0.01 and 0.5 and relative risk to ASD between 1 and 20, because genes with huge effect sizes and larger selection coefficients are expected to be identified from the enrichment of de novo variants. The triangular region where s < 0.013RR are left blank because the parameters in this region are not compatible with the current estimates of prevalence of ASD (1/54)71 and sex-averaged reduction of reproductive fitness (0.71)72. Five new ASD genes identified in this study are placed onto the heatmap closest to its LoF mutation rate. Their positions within heatmaps are taken from point estimates of using gnomAD exomes (non-neuro subset) as population controls.
Extended Data Fig. 7 Sample sizes required for achieving 90% of power.
Using the same assumptions and experiment-wide error rate as power calculation, we calculated required sample size for 90% of power. Sample size is shown as a factor relative to the current sample size (32,024) and as a function of relative risk to ASD and selection coefficient across with different LoF mutation rates. Contours 1, 2 and 5 times of current size are shown as dashed lines. Regions of parameter space that require over 10 times current sample sizes are shown in gray.
Supplementary information
Supplementary Information
Supplementary Note, Supplementary Table Legends, Supplementary Figs. S1–S24, References.
Supplementary Tables
Supplementary Tables S1–S19.
Supplementary Data 1
Annotated de novo coding variants in ASD discovery cohorts.
Supplementary Data 2
Annotated de novo coding variants identified in the SPARK replication cohort.
Supplementary Data 3
Annotated de novo coding variants collected from other NDD studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, X., Feliciano, P., Shu, C. et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat Genet 54, 1305–1319 (2022). https://doi.org/10.1038/s41588-022-01148-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-022-01148-2
This article is cited by
-
Meta-analysis of 46,000 germline de novo mutations linked to human inherited disease
Human Genomics (2024)
-
Olfactory bulb anomalies in KBG syndrome mouse model and patients
BMC Medicine (2024)
-
Clinical impact and in vitro characterization of ADNP variants in pediatric patients
Molecular Autism (2024)
-
Statistical methods for assessing the effects of de novo variants on birth defects
Human Genomics (2024)
-
The complex etiology of autism spectrum disorder due to missense mutations of CHD8
Molecular Psychiatry (2024)