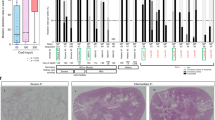
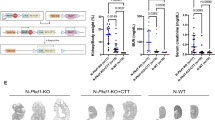
Abstract
Initiation of cyst formation in autosomal dominant polycystic kidney disease (ADPKD) occurs when kidney tubule cells are rendered null for either PKD1 or PKD2 by somatic ‘second hit’ mutations. Subsequent cyst progression remodels the organ through changes in tubule cell shape, proliferation and secretion. The kidney develops inflammation and fibrosis. We constructed a mouse model in which adult inactivation of either Pkd gene can be followed by reactivation of the gene at a later time. Using this model, we show that re-expression of Pkd genes in cystic kidneys results in rapid reversal of ADPKD. Cyst cell proliferation is reduced, autophagy is activated and cystic tubules with expanded lumina lined by squamoid cells revert to normal lumina lined by cuboidal cells. Increases in inflammation, extracellular matrix deposition and myofibroblast activation are reversed, and the kidneys become smaller. We conclude that phenotypic features of ADPKD are reversible and that the kidney has an unexpected capacity for plasticity controlled at least in part by ADPKD gene function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
There are no accession codes, unique identifiers or weblinks for publicly available datasets associated with this publication. The figures do not have associated raw data. There are no restrictions on data availability associated with any part of this work. Full-length western blots supporting all the figures are provided as Source Data.
References
Harris, P. C. & Torres, V. E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Invest. 124, 2315–2324 (2014).
Qian, F., Watnick, T. J., Onuchic, L. F. & Germino, G. G. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87, 979–987 (1996).
Wu, G. et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93, 177–188 (1998).
Leonhard, W. N., Happe, H. & Peters, D. J. Variable cyst development in autosomal dominant polycystic kidney disease: the biologic context. J. Am. Soc. Nephrol. 27, 3530–3538 (2016).
Piontek, K., Menezes, L. F., Garcia-Gonzalez, M. A., Huso, D. L. & Germino, G. G. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13, 1490–1495 (2007).
Lantinga-van Leeuwen, I. S. et al. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum. Mol. Genet. 16, 3188–3196 (2007).
Davenport, J. R. et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586–1594 (2007).
Takakura, A. et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum. Mol. Genet. 18, 2523–2531 (2009).
Patel, V. et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17, 1578–1590 (2008).
Bastos, A. P. et al. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J. Am. Soc. Nephrol. 20, 2389–2402 (2009).
Formica, C. & Peters, D. J. M. Molecular pathways involved in injury-repair and ADPKD progression. Cell Signal. 72, 109648 (2020).
Karihaloo, A. et al. Macrophages promote cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 22, 1809–1814 (2011).
Chen, L. et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J. Clin. Invest. 125, 2399–2412 (2015).
Swenson-Fields, K. I. et al. Macrophages promote polycystic kidney disease progression. Kidney Int. 83, 855–864 (2013).
Cassini, M. F. et al. Mcp1 promotes macrophage-dependent cyst expansion in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 29, 2471–2481 (2018).
Song, C. J., Zimmerman, K. A., Henke, S. J. & Yoder, B. K. Inflammation and fibrosis in polycystic kidney disease. Results Probl. Cell Differ. 60, 323–344 (2017).
Cornec-Le Gall, E. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J. Am. Soc. Nephrol. 24, 1006–1013 (2013).
Fedeles, S. V. et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 43, 639–647 (2011).
Hopp, K. et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J. Clin. Invest. 122, 4257–4273 (2012).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Torres, V. E. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367, 2407–2418 (2012).
Torres, V. E. et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 377, 1930–1942 (2017).
Calvet, J. P. The role of calcium and cyclic AMP in PKD. in Polycystic Kidney Disease (ed. Li, X.) 169-196 (Codon Publications, 2015).
Kakade, V. R. et al. A cAMP and CREB-mediated feed-forward mechanism regulates GSK3beta in polycystic kidney disease. J. Mol. Cell. Biol. 8, 464–476 (2016).
Parnell, S. C. et al. A mutation affecting polycystin-1 mediated heterotrimeric G-protein signaling causes PKD. Hum. Mol. Genet. 27, 3313–3324 (2018).
Zhang, B., Tran, U. & Wessely, O. Polycystin 1 loss of function is directly linked to an imbalance in G-protein signaling in the kidney. Development 145, dev158931 (2018).
Tsiokas, L. Function and regulation of TRPP2 at the plasma membrane. Am. J. Physiol. Renal Physiol. 297, F1–F9 (2009).
Kipp, K. R. et al. Comparison of folate-conjugated rapamycin versus unconjugated rapamycin in an orthologous mouse model of polycystic kidney disease. Am. J. Physiol. Renal Physiol. 315, F395–F405 (2018).
Fantus, D., Rogers, N. M., Grahammer, F., Huber, T. B. & Thomson, A. W. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat. Rev. Nephrol. 12, 587–609 (2016).
Shillingford, J. M., Piontek, K. B., Germino, G. G. & Weimbs, T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J. Am. Soc. Nephrol. 21, 489–497 (2010).
DeCaen, P. G., Delling, M., Vien, T. N. & Clapham, D. E. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318 (2013).
Delling, M., DeCaen, P. G., Doerner, J. F., Febvay, S. & Clapham, D. E. Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314 (2013).
Ha, K. et al. The heteromeric PC-1/PC-2 polycystin complex is activated by the PC-1 N-terminus. eLife 9, e60684 (2020).
Harskamp, L. R., Gansevoort, R. T., van Goor, H. & Meijer, E. The epidermal growth factor receptor pathway in chronic kidney diseases. Nat. Rev. Nephrol. 12, 496–506 (2016).
Bukanov, N. O. et al. CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD. Cell Cycle 11, 4040–4046 (2012).
Lee, K., Boctor, S., Barisoni, L. M. & Gusella, G. L. Inactivation of integrin-beta1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J. Am. Soc. Nephrol. 26, 888–895 (2015).
Raman, A. et al. Integrin-linked kinase signaling promotes cyst growth and fibrosis in polycystic kidney disease. J. Am. Soc. Nephrol. 28, 2708–2719 (2017).
Li, X. Epigenetics in ADPKD: understanding mechanisms and discovering treatment. in Polycystic Kidney Disease (ed. Li, X.) 283-312 (Codon Publications, 2015).
Chang, M. Y. & Ong, A. C. M. Targeting new cellular disease pathways in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 33, 1310–1316 (2017).
Rowe, I. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 (2013).
Chiaravalli, M. et al. 2-Deoxy-d-glucose ameliorates PKD progression. J. Am. Soc. Nephrol. 27, 1958–1969 (2016).
Padovano, V., Podrini, C., Boletta, A. & Caplan, M. J. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat. Rev. Nephrol. 14, 678–687 (2018).
Flowers, E. M. et al. Lkb1 deficiency confers glutamine dependency in polycystic kidney disease. Nat. Commun. 9, 814 (2018).
Kim, S. et al. The polycystin complex mediates Wnt/Ca2+ signalling. Nat. Cell Biol. 18, 752–764 (2016).
Ma, M., Tian, X., Igarashi, P., Pazour, G. J. & Somlo, S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 (2013).
Takiar, V. & Caplan, M. J. Polycystic kidney disease: pathogenesis and potential therapies. Biochim. Biophys. Acta 1812, 1337–1343 (2011).
Avasthi, P., Maser, R.L. & Tran, P.V. in Kidney Development and Disease (ed. Miller, R.K.) 281–321 (Springer International Publishing, 2017).
Ma, M., Gallagher, A. R. & Somlo, S. Ciliary mechanisms of cyst formation in polycystic kidney disease. Cold Spring Harb. Perspect. Biol. 9, a028209 (2017).
Lao, Z., Raju, G. P., Bai, C. B. & Joyner, A. L. MASTR: a technique for mosaic mutant analysis with spatial and temporal control of recombination using conditional floxed alleles in mice. Cell Rep. 2, 386–396 (2012).
Zhang, M. & Kirsch, D. G. The generation and characterization of novel Col1a1FRT-Cre-ER-T2-FRT and Col1a1FRT-STOP-FRT-Cre-ER-T2 mice for sequential mutagenesis. Dis. Model Mech. 8, 1155–1166 (2015).
Nishio, S. et al. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J. Clin. Invest. 115, 910–918 (2005).
Thomson, R. B. et al. Histopathological analysis of renal cystic epithelia in the Pkd2WS25/- mouse model of ADPKD. Am. J. Physiol. Renal Physiol. 285, F870–F880 (2003).
Stroope, A. et al. Hepato-renal pathology in Pkd2ws25/- mice, an animal model of autosomal dominant polycystic kidney disease. Am. J. Pathol. 176, 1282–1291 (2010).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Tang, C., Livingston, M. J., Liu, Z. & Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 16, 489–508 (2020).
Shibazaki, S. et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 17, 1505–1516 (2008).
Torres, J. A. et al. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab. 30, 1007–1023 (2019).
Fragiadaki, M. et al. STAT5 drives abnormal proliferation in autosomal dominant polycystic kidney disease. Kidney Int. 91, 575–586 (2017).
Zerjatke, T. et al. Quantitative cell cycle analysis based on an endogenous all-in-one reporter for cell tracking and classification. Cell Rep. 19, 1953–1966 (2017).
Li, L. X. et al. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J. Clin. Invest. 127, 2751–2764 (2017).
Zhang, C. et al. Cyclin-dependent kinase 1 activity is a driver of cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 32, 41–51 (2021).
Zimmerman, K. A., Hopp, K. & Mrug, M. Role of chemokines, innate and adaptive immunity. Cell Signal. 73, 109647 (2020).
Zheng, D. et al. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 14, 2588–2595 (2003).
Grantham, J. J. et al. Tolvaptan suppresses monocyte chemotactic protein-1 excretion in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 32, 969–975 (2017).
Lannoy, M. et al. The positive effect of selective prostaglandin E2 receptor EP2 and EP4 blockade on cystogenesis in vitro is counteracted by increased kidney inflammation in vivo. Kidney Int. 98, 404–419 (2020).
Lakhia, R. et al. Interstitial microRNA miR-214 attenuates inflammation and polycystic kidney disease progression. JCI Insight 5, e133785 (2020).
Le Hir, M., Hegyi, I., Cueni-Loffing, D., Loffing, J. & Kaissling, B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem. Cell Biol. 123, 335–346 (2005).
Österreicher, C. H. et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl Acad. Sci. USA 108, 308–313 (2011).
Jo, S. K., Sung, S. A., Cho, W. Y., Go, K. J. & Kim, H. K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 21, 1231–1239 (2006).
Durlacher-Betzer, K. et al. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 94, 315–325 (2018).
Kielar, M. L. et al. Maladaptive role of IL-6 in ischemic acute renal failure. J. Am. Soc. Nephrol. 16, 3315–3325 (2005).
Ravichandran, K., Zafar, I., Ozkok, A. & Edelstein, C. L. An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol. Dial. Transplant. 30, 45–53 (2015).
Menon, V. et al. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 7–13 (2011).
Li, X. et al. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 14, 863–868 (2008).
Zhou, J. X., Fan, L. X., Li, X., Calvet, J. P. & Li, X. TNFalpha signaling regulates cystic epithelial cell proliferation through Akt/mTOR and ERK/MAPK/Cdk2 mediated Id2 signaling. PLoS ONE 10, e0131043 (2015).
McGeough, M. D. et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J. Clin. Invest. 127, 4488–4497 (2017).
Jain, N., Sudhakar, C. & Swarup, G. Tumor necrosis factor-alpha-induced caspase-1 gene expression. Role of p73. FEBS J. 274, 4396–4407 (2007).
Alvarez, S. & Munoz-Fernandez, M. A. TNF-alpha may mediate inflammasome activation in the absence of bacterial infection in more than one way. PLoS ONE 8, e71477 (2013).
Mulay, S. R. et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 123, 236–246 (2013).
Knauf, F. et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 84, 895–901 (2013).
Torres, J. A. et al. Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. J. Clin. Invest. 130, 4506–4522 (2019).
Mangos, S. et al. The ADPKD genes Pkd1a/b and Pkd2 regulate extracellular matrix formation. Dis. Model Mech. 3, 354–365 (2010).
Hassane, S. et al. Elevated TGFbeta-Smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J. Pathol. 222, 21–31 (2010).
Dwivedi, N. et al. Epithelial vasopressin type-2 receptors regulate myofibroblasts by a YAP-CCN2-dependent mechanism in polycystic kidney disease. J. Am. Soc. Nephrol. 31, 1697–1710 (2020).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Menezes, L. F., Lin, C. C., Zhou, F. & Germino, G. G. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. eBioMedicine 5, 183–192 (2016).
Zhu, P., Sieben, C. J., Xu, X., Harris, P. C. & Lin, X. Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum. Mol. Genet. 26, 158–172 (2017).
Peintner, L. et al. Loss of PKD1/polycystin-1 impairs lysosomal activity in a CAPN (calpain)-dependent manner. Autophagy https://doi.org/10.1080/15548627.2020.1826716 (2020).
Belibi, F. et al. Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD). Am. J. Physiol. Renal Physiol. 300, F1235–F1243 (2011).
Chou, L. F. et al. Effect of trehalose supplementation on autophagy and cystogenesis in a mouse model of polycystic kidney disease. Nutrients 11, 42 (2018).
Criollo, A. et al. Polycystin-2-dependent control of cardiomyocyte autophagy. J. Mol. Cell. Cardiol. 118, 110–121 (2018).
Praetorius, H. A. The primary cilium as sensor of fluid flow: new building blocks to the model. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol. 308, C198–C208 (2015).
Grantham, J. J. et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 354, 2122–2130 (2006).
Wang, S., Zhao, Y., Leiby, M. & Zhu, J. A new positive/negative selection scheme for precise BAC recombineering. Mol. Biotechnol. 42, 110–116 (2009).
Warming, S., Costantino, N., Court, D. L., Jenkins, N. A. & Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005).
Cai, Y. et al. Altered trafficking and stability of polycystins underlie polycystic kidney disease. J. Clin. Invest. 124, 5129–5144 (2014).
Zou, Z. et al. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J. Biol. Chem. 279, 34302–34310 (2004).
Cai, Y. et al. Identification and characterization of polycystin-2, the PKD2 gene product. J. Biol. Chem. 274, 28557–28565 (1999).
Acknowledgements
We thank the George M. O’Brien Kidney Center at Yale (P30 DK079310) for assistance with BAC recombineering and BUN measurements. This work was supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grants (nos. R01 DK121948 and RC1 DK086738 to S.S.) and a PKD Foundation grant (no. 196F14 to M.M.). We thank the Amy P. Goldman Foundation for their generous and steadfast support.
Author information
Authors and Affiliations
Contributions
K.D., C.Z., X.T. and M.M. performed the experiments. D.C. and F.H. performed and analyzed the MRI studies. S.S. conceived the study. K.D. and S.S. designed the study and analyzed the data. K.D. and S.S. prepared the figures. S.S. wrote the manuscript with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Genetics thanks Bradley Yoder, Michael Köttgen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Note, Tables 1 and 2, and Figs. 1–16 and source data for Supplementary Figures.
Source data
Source Data Fig. 1
Full-length, unprocessed gels or blots.
Source Data Fig. 3
Full-length, unprocessed gels or blots.
Source Data Fig. 4
Full-length, unprocessed gels or blots..
Source Data Fig. 5
Full-length, unprocessed gels or blots.
Source Data Fig. 6
Full-length, unprocessed gels or blots.
Rights and permissions
About this article
Cite this article
Dong, K., Zhang, C., Tian, X. et al. Renal plasticity revealed through reversal of polycystic kidney disease in mice. Nat Genet 53, 1649–1663 (2021). https://doi.org/10.1038/s41588-021-00946-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-021-00946-4
This article is cited by
-
AI models for automated segmentation of engineered polycystic kidney tubules
Scientific Reports (2024)
-
Exploring the clinical and genetical spectrum of ADPKD in Chile to assess ProPKD score as a risk prediction tool
Translational Medicine Communications (2023)
-
1-Indanone retards cyst development in ADPKD mouse model by stabilizing tubulin and down-regulating anterograde transport of cilia
Acta Pharmacologica Sinica (2023)
-
The C-terminal tail of polycystin-1 suppresses cystic disease in a mitochondrial enzyme-dependent fashion
Nature Communications (2023)
-
Primary cilia as dynamic and diverse signalling hubs in development and disease
Nature Reviews Genetics (2023)