Abstract
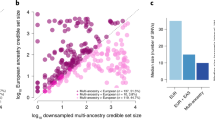
Glycemic traits are used to diagnose and monitor type 2 diabetes and cardiometabolic health. To date, most genetic studies of glycemic traits have focused on individuals of European ancestry. Here we aggregated genome-wide association studies comprising up to 281,416 individuals without diabetes (30% non-European ancestry) for whom fasting glucose, 2-h glucose after an oral glucose challenge, glycated hemoglobin and fasting insulin data were available. Trans-ancestry and single-ancestry meta-analyses identified 242 loci (99 novel; P < 5 × 10−8), 80% of which had no significant evidence of between-ancestry heterogeneity. Analyses restricted to individuals of European ancestry with equivalent sample size would have led to 24 fewer new loci. Compared with single-ancestry analyses, equivalent-sized trans-ancestry fine-mapping reduced the number of estimated variants in 99% credible sets by a median of 37.5%. Genomic-feature, gene-expression and gene-set analyses revealed distinct biological signatures for each trait, highlighting different underlying biological pathways. Our results increase our understanding of diabetes pathophysiology by using trans-ancestry studies for improved power and resolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Ancestry-specific and overall meta-analysis summary level results are available through the MAGIC website (https://www.magicinvestigators.org/). Summary statistics are also available through the GWAS catalog (https://www.ebi.ac.uk/gwas/) with the following accession codes: GCST90002225, GCST90002226, GCST90002227, GCST90002228, GCST90002229, GCST90002230, GCST90002231, GCST90002232, GCST90002233, GCST90002234, GCST90002235, GCST90002236, GCST90002237, GCST90002238, GCST90002239, GCST90002240, GCST90002241, GCST90002242, GCST90002243, GCST90002244, GCST90002245, GCST90002246, GCST90002247 and GCST90002248.
Code availability
Source code implementing the methods described in the paper are publicly available at https://doi.org/10.5281/zenodo.4607311.
References
Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation Report No. WHO/NMH/CHP/CPM/11.1 (World Health Organization, 2011).
Goodarzi, M. O. et al. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am. J. Physiol. Endocrinol. Metab. 301, E402–E408 (2011).
Dimas, A. S. et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 63, 2158–2171 (2014).
Udler, M. S. et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 15, e1002654 (2018).
Udler, M. S., McCarthy, M. I., Florez, J. C. & Mahajan, A. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr. Rev. 40, 1500–1520 (2019).
The Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375, 2215–2222 (2010).
Wheeler, E. et al. Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome-wide meta-analysis. PLoS Med. 14, e1002383 (2017).
Dupuis, J. et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42, 105–116 (2010).
Manning, A. K. et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 44, 659–669 (2012).
Walford, G. A. et al. Genome-wide association study of the modified Stumvoll insulin sensitivity index identifies BCL2 and FAM19A2 as novel insulin sensitivity loci. Diabetes 65, 3200–3211 (2016).
Horikoshi, M. et al. Discovery and fine-mapping of glycaemic and obesity-related trait loci using high-density imputation. PLoS Genet. 11, e1005230 (2015).
Mahajan, A. et al. Identification and functional characterization of G6PC2 coding variants influencing glycemic traits define an effector transcript at the G6PC2-ABCB11 locus. PLoS Genet. 11, e1004876 (2015).
Hwang, J. Y. et al. Genome-wide association meta-analysis identifies novel variants associated with fasting plasma glucose in East Asians. Diabetes 64, 291–298 (2015).
Chen, P. et al. Multiple nonglycemic genomic loci are newly associated with blood level of glycated hemoglobin in East Asians. Diabetes 63, 2551–2562 (2014).
Scott, R. A. et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat. Genet. 44, 991–1005 (2012).
Spanakis, E. K. & Golden, S. H. Race/ethnic difference in diabetes and diabetic complications. Curr. Diabetes Rep. 13, 814–823 (2013).
Tillin, T. et al. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall And Brent REvisited (SABRE) cohort. Diabetes Care 36, 383–393 (2013).
Whincup, P. H. et al. Early emergence of ethnic differences in type 2 diabetes precursors in the UK: the Child Heart and Health Study in England (CHASE Study). PLoS Med. 7, e1000263 (2010).
The 1000 Genomes Project Consortium A global reference for human genetic variation. Nature 526, 68–74 (2015).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Mahajan, A. et al. Trans-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Preprint at medRxiv https://doi.org/10.1101/2020.09.22.20198937 (2020).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Spracklen, C. N. et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature 582, 240–245 (2020).
Vujkovic, M. et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 52, 680–691 (2020).
Luo, Y. et al. Transcription factor Ets1 regulates expression of thioredoxin-interacting protein and inhibits insulin secretion in pancreatic beta-cells. PLoS ONE 9, e99049 (2014).
Braccini, L. et al. PI3K-C2γ is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat. Commun. 6, 7400 (2015).
Aschard, H., Vilhjálmsson, B. J., Joshi, A. D., Price, A. L. & Kraft, P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am. J. Hum. Genet. 96, 329–339 (2015).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Nolte, I. M. et al. Missing heritability: is the gap closing? An analysis of 32 complex traits in the Lifelines Cohort Study. Eur. J. Hum. Genet. 25, 877–885 (2017).
Ge, T., Chen, C. Y., Ni, Y., Feng, Y. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1776 (2019).
Dastani, Z. et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 8, e1002607 (2012).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51, 584–591 (2019).
Gaulton, K. J. et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat. Genet. 47, 1415–1425 (2015).
Spracklen, C. N. et al. Identification and functional analysis of glycemic trait loci in the China Health and Nutrition Survey. PLoS Genet. 14, e1007275 (2018).
Varshney, A. et al. Genetic regulatory signatures underlying islet gene expression and type 2 diabetes. Proc. Natl Acad. Sci. USA 114, 2301–2306 (2017).
Kichaev, G. et al. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104, 65–75 (2019).
Shriner, D. & Rotimi, C. N. Whole-genome-sequence-based haplotypes reveal single origin of the sickle allele during the Holocene wet phase. Am. J. Hum. Genet. 102, 547–556 (2018).
Kramer, H. J. et al. African ancestry-specific alleles and kidney disease risk in Hispanics/Latinos. J. Am. Soc. Nephrol. 28, 915–922 (2017).
Ravenhall, M. et al. Novel genetic polymorphisms associated with severe malaria and under selective pressure in North-eastern Tanzania. PLoS Genet. 14, e1007172 (2018).
Hodonsky, C. J. et al. Genome-wide association study of red blood cell traits in Hispanics/Latinos: The Hispanic Community Health Study/Study of Latinos. PLoS Genet. 13, e1006760 (2017).
Gurdasani, D. et al. Uganda genome resource enables insights into population history and genomic discovery in Africa. Cell 179, 984–1002 (2019).
Rees, M. G. et al. Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia 55, 114–122 (2012).
Bonomo, J. A. et al. The ras responsive transcription factor RREB1 is a novel candidate gene for type 2 diabetes associated end-stage kidney disease. Hum. Mol. Genet. 23, 6441–6447 (2014).
Wessel, J. et al. Low-frequency and rare exome chip variants associate with fasting glucose and type 2 diabetes susceptibility. Nat. Commun. 6, 5897 (2015).
Scott, R. A. et al. A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci. Transl. Med. 8, 341ra76 (2016).
Nai, A. et al. TMPRSS6 rs855791 modulates hepcidin transcription in vitro and serum hepcidin levels in normal individuals. Blood 118, 4459–4462 (2011).
Ng, N. H. J. et al. Tissue-specific alteration of metabolic pathways influences glycemic regulation. Preprint at bioRxiv https://doi.org/10.1101/790618 (2019).
Soranzo, N. et al. Common variants at 10 genomic loci influence hemoglobin A1C levels via glycemic and nonglycemic pathways. Diabetes 59, 3229–3239 (2010).
Sarnowski, C. et al. Impact of rare and common genetic variants on diabetes diagnosis by hemoglobin A1c in multi-ancestry cohorts: the trans-omics for precision medicine program. Am. J. Hum. Genet. 105, 706–718 (2019).
Kundaje, A. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Nagel, M. et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 50, 920–927 (2018).
Savage, J. E. et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50, 912–919 (2018).
Schmidt, E. M. et al. GREGOR: evaluating global enrichment of trait-associated variants in epigenomic features using a systematic, data-driven approach. Bioinformatics 31, 2601–2606 (2015).
Parker, S. C. et al. Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Proc. Natl Acad. Sci. USA 110, 17921–17926 (2013).
Pickrell, J. K. Joint analysis of functional genomic data and genome-wide association studies of 18 human traits. Am. J. Hum. Genet. 94, 559–573 (2014).
Iotchkova, V. et al. GARFIELD classifies disease-relevant genomic features through integration of functional annotations with association signals. Nat. Genet. 51, 343–353 (2019).
van de Bunt, M. et al. Transcript expression data from human islets links regulatory signals from genome-wide association studies for type 2 diabetes and glycemic traits to their downstream effectors. PLoS Genet. 11, e1005694 (2015).
Civelek, M. et al. Genetic regulation of adipose gene expression and cardio-metabolic traits. Am. J. Hum. Genet. 100, 428–443 (2017).
Scott, L. J. et al. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 7, 11764 (2016).
Ben Harouch, S., Klar, A. & Falik Zaccai, T. C. in GeneReviews (eds Adam, M. P. et al.) (Univ. of Washington, 1993).
Agus, D. B. et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 100, 2842–2848 (1997).
Wolking, S. et al. Focal epilepsy in glucose transporter type 1 (Glut1) defects: case reports and a review of literature. J. Neurol. 261, 1881–1886 (2014).
Guallar, D. et al. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat. Genet. 50, 443–451 (2018).
Bian, F. et al. TET2 facilitates PPARγ agonist-mediated gene regulation and insulin sensitization in adipocytes. Metabolism 89, 39–47 (2018).
Yoo, Y. et al. TET-mediated hydroxymethylcytosine at the Pparγ locus is required for initiation of adipogenic differentiation. Int. J. Obes. 41, 652–659 (2017).
Lees, J. A. et al. Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science 355, eaah6171 (2017).
Pottekat, A. et al. Insulin biosynthetic interaction network component, TMEM24, facilitates insulin reserve pool release. Cell Rep. 4, 921–930 (2013).
Androulakis, I. I. et al. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J. Clin. Endocrinol. Metab. 99, 2754–2762 (2014).
Altieri, B. et al. Adrenocortical tumors and insulin resistance: what is the first step? Int. J. Cancer 138, 2785–2794 (2016).
Johansson, M. et al. The influence of obesity-related factors in the etiology of renal cell carcinoma-A Mendelian randomization study. PLoS Med. 16, e1002724 (2019).
Diamanti-Kandarakis, E. & Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33, 981–1030 (2012).
The DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–990 (2012).
Leong, A. et al. Mendelian randomization analysis of hemoglobin A1C as a risk factor for coronary artery disease. Diabetes Care 42, 1202–1208 (2019).
Duncan, L. et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 10, 3328 (2019).
Mostafavi, H. et al. Variable prediction accuracy of polygenic scores within an ancestry group. eLife 9, e48376 (2020).
Choi, S. W., Mak, T. S. & O’Reilly, P. F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 15, 2759–2772 (2020).
D’Orazio, P. et al. Approved IFCC recommendation on reporting results for blood glucose (abbreviated). Clin. Chem. 51, 1573–1576 (2005).
Voight, B. F. et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 8, e1002793 (2012).
The 1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Li, Y., Willer, C. J., Ding, J., Scheet, P. & Abecasis, G. R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 34, 816–834 (2010).
Pei, Y. F., Zhang, L., Li, J. & Deng, H. W. Analyses and comparison of imputation-based association methods. PLoS ONE 5, e10827 (2010).
Winkler, T. W. et al. Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc. 9, 1192–1212 (2014).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Morris, A. P. Transethnic meta-analysis of genomewide association studies. Genet. Epidemiol. 35, 809–822 (2011).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Benner, C. et al. FINEMAP: efficient variable selection using summary data from genome-wide association studies. Bioinformatics 32, 1493–1501 (2016).
Astle, W. J. et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 167, 1415–1429 (2016).
Canela-Xandri, O., Rawlik, K. & Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 50, 1593–1599 (2018).
Benyamin, B. et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat. Commun. 5, 4926 (2014).
Binesh, N. & Rezghi, M. Fuzzy clustering in community detection based on nonnegative matrix factoriztion with two novel evaluation criteria. Appl. Soft Comput. 69, 689–703 (2018).
Scott, R. A. et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 66, 2888–2902 (2017).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Mikkelsen, T. S. et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell 143, 156–169 (2010).
Ernst, J. & Kellis, M. ChromHMM: automating chromatin-state discovery and characterization. Nat. Methods 9, 215–216 (2012).
GTEx Consortium Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
Zhernakova, D. V. et al. Identification of context-dependent expression quantitative trait loci in whole blood. Nat. Genet. 49, 139–145 (2017).
Westra, H. J. et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 45, 1238–1243 (2013).
Joehanes, R. et al. Integrated genome-wide analysis of expression quantitative trait loci aids interpretation of genomic association studies. Genome Biol. 18, 16 (2017).
Pers, T. H. et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 6, 5890 (2015).
Acknowledgements
We thank all investigators, staff members and study participants for their contribution to all participating studies. The funders had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript. The authors received no specific funding for this work. A full list of funding as well as individual and study acknowledgments appears in the Supplementary Note.
Author information
Authors and Affiliations
Consortia
Contributions
Project coordination: I.B. Writing group: J.C., C.N.S., G. Marenne, A.V., L.J.C., S.C.J.P., K.L.M., C. Langenberg, E.W., A.P.M. and I.B. Central analysis group: J.C., C.N.S., G. Marenne, A.V., L.J.C, J’an Luan, S.W., Y. Wu, X.Z., M.H., T.S.B., R.M., J.W., A.P., R.L.-G., K.H.K.C., J.Y., M.D.A., A.Y.C., A. Claringbould, J. Heikkinen, J. Hong, J.-J.H., S. Huo, M.A.K., T.L., W.M., H.M.-M., A. Ndungu, S.C.N., K.N., C.K.R., D. Ray, R. Rohde, D. Rybin, C. Schurmann, X.S., L.S., I.D.S., C.A.W., Y. Wang, P.W., W. Zhang, J.I.R., A.L.G., M.I.M., J.D., J.B.M., R.A.S., I.P., A. Leong, C.-T.L., S.C.J.P., K.L.M., C. Langenberg, E.W., A.P.M. and I.B. Cohort analysts: T.S.A., E.V.R.A., L.F.B., J.A.B., N.P.B., C.P.C., B.E.C., J.C., X.C., L.-C.C., C.-H.C., B.H.C., K.C., Y.-F.C., H.G.d.H., G.E.D., A. Demirkan, Q.D., J.E., S.A.F., J.G., F.G., J.G., S. Gustafsson, Y. Hai, F.P.H., J.-J.H., Y. Heianza, T. Huang, A.H.-C., M.H., R.A.J., T. Kawaguchi, K.A.K., Y.K., M.E.K., I.K.K., S. Lai, L.A.L., C.D.L., M. Lauzon, M. Li, S. Ligthart, J. Liu, M. Loh, J. Long, V.L., M.M., C.M., M.E.M., A. Nag, M. Nakatochi, D.N., R.N., G.P., M.P., L.R., L.J.R.-T., S.S.R., N.R.R., R. Rueedi, K. Ryan, S.S., R.S., K.E.S., B.S., J. He, K. Setoh, A.V.S., L.S., T. Sparsø, R.J.S., F.T., J. Tan, S.T., E.v.d.A., P.J.v.d.M., N.V., M.V., H. Wang, C.W., N.W., H.R.W., W.W., T. Wilsgaard, A.W., A. R. Wood, T.X., M.Z., J.-H.Z. and W. Zhao. Cohort genotyping and phenotyping: N.A., Z.A., A.A., S.J.L.B., D.B., M. Beekman, R.N.B., A.B., M. Blüher, L.L.B., S.R.B., D.W.B., Q.C., A. Campbell, H.C., Y.-F.C., E.J.C.d.G., A. Dehghan, S.D., G.E., A.F., M.F., C.F., Y.G., A.P.G., A.G., S. Han, C.A.H., C.-H.H., A.A.H., C. Herder, Y.C.C., W.A.H., S.I., M.I., M.A.I., W.C.J., M.E.J., P.K.J., R.R.K., F.R.K., T. Katsuya, C.K., W.K., I.K., T. Kuulasmaa, J.K., K. Läll, K. Lam, D.A.L., N.R.L., R.N.L., Honglan Li, S.-Y.L., J. Lindström, A. Linneberg, J. Liu, C. Lorenzo, T.M., F.M., G. Mingrone, S.M., S.M., T.N., G.N.N., J.L.N., M. Nelis, M.J.N., J.M.N., Y.O., A.P., P.A.P., O. Polasek, Q.Q., D. Raven, D.F.R., A.R., F.R., K. Roll, I.R., C. Sabanayagam, K. Sandow, N. Sattar, A. Schürmann, J. Shi, H.M.S., K.D.T., T.M.T., B.T., P.R.H.J.T., E.T., M.Y.T., A.U., R.M.v.D., D.v.H., A.v.H.V., J.V.v.V.-O., J.V., H.V., T. Wang, T.-Y.W., K.W.v.D. and T.Z. Cohort oversight and/or principal investigator: G.R.A., L.S.A., C.A.A.-S., M.E.A.-R., P.A., L.A.-S., D.M.B., L.J.B., S.B., H.B., C.B., M. Boehnke, E.B., B.O.B., K.B., D.I.B., E.P.B., T.A.B., M.C., M.J.C., J.C.C., D.I.C., Y.-D.I.C., C.-Y.C., F.S.C., A. Correa, F.C., H.G.d.H., G.D., S.E., M.K.E., E.F., L.F., J.C.F., P.W.F., T.M.F., P.F., B.G., M.O.G., P.G.-L., H.G., N.G., S. Grimsgaard, L.G., V.G., X.G., A.H., T. Hansen, C. Hayward, S.R.H., B.L.H., W.H., E.I., P.S.J., M.-R.J., J.B.J., J.W.J., P. Kaleebu, R.K., S.L.R.K., N.K., S.M.K.-K., B.-J.K., M. Kivimaki, H.A.K., J.S.K., A.K., P. Kovacs, D.K., M. Kumari, Z.K., M. Laakso, T.A.L., L.J.L., K. Leander, Huaixing Li, X.L., L.L., C. Lindgren, S. Liu, R.J.F.L., P.K.E.M., A. Mahajan, A. Metspalu, D.O.M.-K., T.A.M., P.B.M., I.N., J.R.O., A.J.O., K.K.O., S.P., C.N.A.P., N.D.P., O. Pedersen, C.E.P., D.J.P., P.P.P., M.A.P., B.M.P., L.Q., L.J.R., R. Rauramaa, S.R., P.M.R., F.R.R., T.E.S., M. Sandhu, J. Saramies, N. Schneiderman, P. Schwarz, L.J.S., E.S., P. Sever, X-o.S., P.E.S., K.S.S., B.H.S., H.S., T. Sofer, T.I.A.S., T.D.S., A. Stanton, C.J.S., M. Stumvoll, Y.T., E.T., N.J.T., A.T., J. Tuomilehto, T.T., M.U., P.v.d.H., C.v.D., P.V., T.G.M.V., L.E.W., M.W., Y.X.W., N.J.W., R.M.W., H. Watkins, W.B.W., A. R. Wickremasinghe, G.W., J.F.W., T.-Y.W., J.-Y.W., A.H.X., L.R.Y., L.Y., M.Y., E.Z., W. Zheng, A.B.Z., J.I.R., A.L.G., M.I.M., J.D., J.B.M., R.A.S., I.P., A.L., C.-T.L., S.C.J.P., K.L.M., C. Langenberg, E.W., A.P.M. and I.B. All authors read, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.A. is the recipient of honoraria as a speaker for a wide range of Danish and international concerns and receives royalties from textbooks, and from popular diet and cookery books. A.A. is also co-inventor of a number of patents, including methods of inducing weight loss, treating obesity and preventing weight gain (licensee Gelesis) and biomarkers for predicting the degree of weight loss (licensee Nestec), owned by the University of Copenhagen, in accordance with Danish law. I.B. and spouse own stock in GlaxoSmithKline and Incyte Corporation. B.H.C. is now an employee of Life Epigenetics; all work was completed before employment by Life Epigenetics. A.Y.C. is now an employee of Merck & Co.; all work was completed before employment by Merck & Co. J.C.F. has received consulting honoraria from Janssen. J.G. is now an employee of F. Hoffmann-La Roche, and owns stock in Roche and GlaxoSmithKline. A.L.G. has received honoraria from Merck and Novo Nordisk. As of June 2019, A.L.G. discloses that her spouse is an employee of Genentech and hold stock options in Roche. E.I. is now an employee of GlaxoSmithKline; all work was completed before his employment by GlaxoSmithKline. W.M. has received grants and/or personal fees from the following companies/corporations: Siemens Healthineers, Aegerion Pharmaceuticals, AMGEN, AstraZeneca, Sanofi, Alexion Pharmaceuticals, BASF, Abbott Diagnostics Numares, Berlin-Chemie, Akzea Therapeutics, Bayer Vital, Bestbion dx, Boehringer Ingelheim Pharma, Immundiagnostik, Merck Chemicals, MSD Sharp and Dohme, Novartis Pharma, Olink Proteomics and Synlab Holding Deutschland. M.I.M. has served on advisory panels for Pfizer, NovoNordisk and Zoe Global, and has received honoraria from Merck, Pfizer, NovoNordisk and Eli Lilly. He holds stock options in Zoe Global and has received research funding from Abbvie, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, NovoNordisk, Pfizer, Roche, Sanofi Aventis, Servier and Takeda. He is now an employee of Genentech and a holder of Roche stock. J.B.M. has consulted for Quest Diagnostics, who is a manufacturer of an HbA1c assay. M.E.M. has received grant funding from Regeneron Pharmaceuticals. M.E.M. is also an inventor on a patent that was published by the US Patent and Trademark Office on 6 December 2018 under Publication Number US 2018-0346888, and international patent application that was published on 13 December 2018 under Publication Number WO-2018/226560; all work was completed before these competing interests arose, and are unrelated to this work. D.O.M.-K. is a part-time clinical research consultant for Metabolon. J.L.N. is a member of the Scientific Advisory Board for Veralox Therapeutics. C.N.A.P. has received research support from GlaxoSmithKline and AstraZeneca unrelated to this project. B.M.P. serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. N. Sattar has consulted for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Napp and Sanofi, and received grant support from Boehringer Ingelheim. R.A.S. is an employee and shareholder of GlaxoSmithKline. T.D.S. is the founder of Zoe Global. J. Tuomilehto receives research support from Bayer, is a consultant for Eli Lily and holds stock in Orion Pharma and Aktivolabs.
Additional information
Peer review information Nature Genetics thanks Anurag Verma and Constantin Polychronakos for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Flow diagram of this study.
The figure shows the data, key methods and main analyses included in this effort.
Extended Data Fig. 2 Locus diagram.
Trans-ancestry locus A contains a trans-ancestry lead variant for one glycemic trait represented by the blue diamond, and another single-ancestry index variant for another glycemic trait represented by the orange triangle. Single-ancestry locus B contains a single-ancestry lead variant represented by the purple square. The orange, blue and purple bars represent a +/− 500Kb window around the orange, blue, and purple variants, respectively. The black bars indicate the full locus window where trans-ancestry locus A contains trans-ancestry lead and single-ancestry index variants for two traits and single-ancestry locus B has a single-ancestry lead variant for a single trait.
Extended Data Fig. 3 Venn diagram.
Overlap of TA loci between traits.
Extended Data Fig. 4 Allele frequency versus effect size.
Allele frequency versus effect size for all signals detected through the trans-ancestry meta-analyses, for each of the four traits. Frequency and effect size are from the European meta-analyses. The power curves were computed based on the European sample size for each trait, and the mean (m) and standard deviation (sd) computed on the FENLAND study: FG, m = 4.83 mmol/l, sd=0.68; FI, m = 3.69 mmol/l, sd=0.60; 2hGlu, m = 5.30 mmol/l, sd=1.74; HbA1c, m = 5.55%, sd=0.48.
Extended Data Fig. 5 EAF correlation and heterogeneity test.
Pearson correlation of EAF on the lower tri-angle and p-value of one-side heterogeneity test without multiple testing corrections on the upper tri-angle of the trans-ancestry lead variants associated with each trait between ancestries. Correlations > 0.7 are in bold.
Extended Data Fig. 6 Forest plot of T2D GRS from HbA1c variants.
The p-value on the right side is from the two-side test without multiple testing corrections. Vertical points of each diamond represent the point estimate of the odds ratio. The horizontal points of each diamond represent the 95% confidence interval of the odds ratio. Figure shows the association results between HbA1c-associated variants built into a GRS for T2D by taking each HbA1c-associated variant and using a weight that corresponds to its T2D effect size (logOR) based on analysis by the DIAGRAM consortium. The overall GRS is subsequently partitioned according to the HbA1c signal classification. The overall and partitioned GRS were tested for association with T2D based on data from UK biobank.
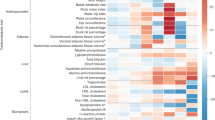
Extended Data Fig. 7 Enrichment of glycemic trait associated GWAS variants to overlap genomic annotations using GREGOR.
Figure shows enrichment for 59 total static and stretch enhancer annotations considered. One-side test significance (red) is determined after Bonferroni correction to account for 59 total annotations tested for each trait; nominal significance (P < 0.05) is indicated in yellow.
Extended Data Fig. 8 Enrichment of glycemic trait associated GWAS variants to overlap genomic annotations using fGWAS.
Figure shows log2(Fold Enrichment) of GWAS variants to overlap 59 static and stretch enhancer annotations calculated. Significant enrichment (red) is considered if the 95% confidence intervals (shown by the error bars) do not overlap 0.
Extended Data Fig. 9 Enrichment of glycemic trait associated GWAS variants to overlap genomic annotations using GARFIELD.
Figure shows the β or effect size (log odds ratio) for GWAS variants to overlap 59 static and stretch enhancer annotations. GWAS variants were included at two significance thresholds, 1e-05 (A) and 1e-08 (B). One-side test significance (red) is determined after Bonferroni correction to account for effective annotations tested for each trait reported by GARFIELD (see Supplementary Note); nominal significance (P < 0.05) is indicated in yellow. The 95% confidence intervals are shown by the error bars.
Supplementary information
Supplementary Information
Supplementary Note
Supplementary Table 1
Supplementary Tables 1–21
Rights and permissions
About this article
Cite this article
Chen, J., Spracklen, C.N., Marenne, G. et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet 53, 840–860 (2021). https://doi.org/10.1038/s41588-021-00852-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-021-00852-9
This article is cited by
-
Causal relationship between diabetes mellitus, glycemic traits and Parkinson’s disease: a multivariable mendelian randomization analysis
Diabetology & Metabolic Syndrome (2024)
-
Joint analysis of mutational and transcriptional landscapes in human cancer reveals key perturbations during cancer evolution
Genome Biology (2024)
-
Associations between type 1 diabetes and pulmonary tuberculosis: a bidirectional mendelian randomization study
Diabetology & Metabolic Syndrome (2024)
-
Causal effects of glycemic traits and endometriosis: a bidirectional and multivariate mendelian randomization study
Diabetology & Metabolic Syndrome (2024)
-
Multi-trait GWAS for diverse ancestries: mapping the knowledge gap
BMC Genomics (2024)