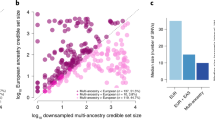
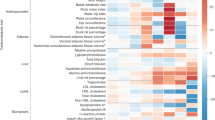
Abstract
We investigated type 2 diabetes (T2D) genetic susceptibility via multi-ancestry meta-analysis of 228,499 cases and 1,178,783 controls in the Million Veteran Program (MVP), DIAMANTE, Biobank Japan and other studies. We report 568 associations, including 286 autosomal, 7 X-chromosomal and 25 identified in ancestry-specific analyses that were previously unreported. Transcriptome-wide association analysis detected 3,568 T2D associations with genetically predicted gene expression in 687 novel genes; of these, 54 are known to interact with FDA-approved drugs. A polygenic risk score (PRS) was strongly associated with increased risk of T2D-related retinopathy and modestly associated with chronic kidney disease (CKD), peripheral artery disease (PAD) and neuropathy. We investigated the genetic etiology of T2D-related vascular outcomes in the MVP and observed statistical SNP–T2D interactions at 13 variants, including coronary heart disease (CHD), CKD, PAD and neuropathy. These findings may help to identify potential therapeutic targets for T2D and genomic pathways that link T2D to vascular outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The full summary-level association data from the trans-ancestry, European, African American, Hispanic and Asian meta-analysis from this report are available through dbGAP under accession number phs001672.v3.p1 (Veterans Administration Million Veteran Program Summary Results from Omics Studies). Source data are provided with this paper. More specifically, dbGaP accession number pha004943.1 refers to the African American–specific summary statistics, pha004944.1 to the Asian-specific summary statistics, pha004945.1 refers to the European-specific summary statistics, pha004946.1 refers to the Hispanic-specific summary statistics, and pha004947.1 refers to the trans-ancestry summary statistics.
Code availability
Imputation was performed using MiniMac4 and EAGLE v2. Association analysis was performed using PLINK2A and XWAS v3.0. Post-GWAS processing software include: PRSice-2, LD Hub v1.9.3, FlashPCA v2.0, METAL v2011-03-25, GCTA-COJO v1.93, S-PrediXcan v0.6.1, SUGEN v8.9, DEPICT v140721, SIDER v4.1, DGIdb v3.0 and KING v2.1.6, as outlined in the Methods. Clear code for analysis is available at the associated website of each software package. Additional analyses were performed in R-3.2.
References
International Diabetes Federation. IDF Diabetes Atlas 8th edn (International Diabetes Federation, 2017).
American Diabetes Association Standards of medical care in diabetes—2018. Diabetes Care 41, S1–S2 (2018).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Suzuki, K. et al. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat. Genet. 51, 379–386 (2019).
Gaziano, J. M. et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 70, 214–223 (2016).
Levin, M. G. et al. Genomic risk stratification predicts all-cause mortality after cardiac catheterization. Circ. Genom. Precis. Med. 11, e002352 (2018).
Saleheen, D. et al. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature 544, 235–239 (2017).
Berglund, G., Elmstahl, S., Janzon, L. & Larsson, S. A. The Malmo Diet and Cancer Study. Design and feasibility. J. Intern. Med. 233, 45–51 (1993).
Reilly, M. P. et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 377, 383–392 (2011).
Bonas-Guarch, S. et al. Re-analysis of public genetic data reveals a rare X-chromosomal variant associated with type 2 diabetes. Nat. Commun. 9, 321 (2018).
Xue, A. et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 9, 2941 (2018).
Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
Luo, Y. et al. Estimating heritability of complex traits in admixed populations with summary statistics. Preprint at bioRxiv https://doi.org/10.1101/503144 (2018).
Klarin, D. et al. Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat. Genet. 50, 1514–1523 (2018).
Kuhn, M., Letunic, I., Jensen, L. J. & Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 44, D1075–D1079 (2016).
Finucane, H. K. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
Schmidt, E. M. et al. GREGOR: evaluating global enrichment of trait-associated variants in epigenomic features using a systematic, data-driven approach. Bioinformatics 31, 2601–2606 (2015).
Ng, M. C. et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet. 10, e1004517 (2014).
Chen, J. et al. Genome-wide association study of type 2 diabetes in Africa. Diabetologia 62, 1204–1211 (2019).
Palmer, N. D. et al. A genome-wide association search for type 2 diabetes genes in African Americans. PLoS ONE 7, e29202 (2012).
Wheeler, E. et al. Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome-wide meta-analysis. PLoS Med. 14, e1002383 (2017).
Mahajan, A. et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 50, 559–571 (2018).
Flannick, J. et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature 570, 71–76 (2019).
Carrano, A. C., Mulas, F., Zeng, C. & Sander, M. Interrogating islets in health and disease with single-cell technologies. Mol. Metab. 6, 991–1001 (2017).
Martin, C. K., Han, H., Anton, S. D., Greenway, F. L. & Smith, S. R. Effect of valproic acid on body weight, food intake, physical activity and hormones: results of a randomized controlled trial. J. Psychopharmacol. 23, 814–825 (2009).
Buse, M. et al. Expanding the phenotype of reciprocal 1q21.1 deletions and duplications: a case series. Ital. J. Pediatr. 43, 61 (2017).
Devi, R. R. & Vijayalakshmi, P. Novel mutations in GJA8 associated with autosomal dominant congenital cataract and microcornea. Mol. Vis. 12, 190–195 (2006).
Mackay, D. S., Bennett, T. M., Culican, S. M. & Shiels, A. Exome sequencing identifies novel and recurrent mutations in GJA8 and CRYGD associated with inherited cataract. Hum. Genomics 8, 19 (2014).
Luo, J. et al. TCF7L2 variation and proliferative diabetic retinopathy. Diabetes 62, 2613–2617 (2013).
Eiden, L. E., Schafer, M. K., Weihe, E. & Schutz, B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 447, 636–640 (2004).
Sharma, P. & Sharma, R. Toxic optic neuropathy. Indian J. Ophthalmol 59, 137–141 (2011).
Pattaro, C. et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 7, 10023 (2016).
Canela-Xandri, O., Rawlik, K. & Tenesa, A. An atlas of genetic associations in UK Biobank. Nat. Genet. 50, 1593–1599 (2018).
Ehret, G. B. et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 48, 1171–1184 (2016).
Sung, Y. J. et al. A large-scale multi-ancestry genome-wide study accounting for smoking behavior identifies multiple significant loci for blood pressure. Am. J. Hum. Genet. 102, 375–400 (2018).
Maass, P. G. et al. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat. Genet. 47, 647–653 (2015).
Shin, S. et al. CREB mediates the insulinotropic and anti-apoptotic effects of GLP-1 signaling in adult mouse β-cells. Mol. Metab 3, 803–812 (2014).
Omar, B., Banke, E., Ekelund, M., Frederiksen, S. & Degerman, E. Alterations in cyclic nucleotide phosphodiesterase activities in omental and subcutaneous adipose tissues in human obesity. Nutr. Diabetes 1, e13 (2011).
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
Fang, H. et al. Harmonizing genetic ancestry and self-identified race/ethnicity in genome-wide association studies. Am. J. Hum. Genet. 105, 763–772 (2019).
Tirschwell, D. L. & Longstreth, W. T. Jr. Validating administrative data in stroke research. Stroke 33, 2465–2470 (2002).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Gao, F. et al. XWAS: a software toolset for genetic data analysis and association studies of the X chromosome. J. Hered. 106, 666–671 (2015).
Ko, Y. A. et al. Genetic-variation-driven gene-expression changes highlight genes with important functions for kidney disease. Am. J. Hum. Genet. 100, 940–953 (2017).
Ackermann, A. M., Wang, Z., Schug, J., Naji, A. & Kaestner, K. H. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 5, 233–244 (2016).
International HapMap Consortium et al. Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Roadmap Epigenomics Consortium et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Andersson, R. et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 (2014).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50, 621–629 (2018).
Fehrmann, R. S. et al. Gene expression analysis identifies global gene dosage sensitivity in cancer. Nat. Genet. 47, 115–125 (2015).
Cahoy, J. D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Heng, T. S. & Painter, M. W., Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Denny, J. C. et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics 26, 1205–1210 (2010).
Voorman, A., Lumley, T., McKnight, B. & Rice, K. Behavior of QQ-plots and genomic control in studies of gene-environment interaction. PLoS ONE 6, e19416 (2011).
Lin, D. Y. et al. Genetic association analysis under complex survey sampling: the Hispanic Community Health Study/Study of Latinos. Am. J. Hum. Genet. 95, 675–688 (2014).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Pers, T. H. et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 6, 5890 (2015).
Acknowledgements
This research is based on data from the MVP, Office of Research and Development, Veterans Health Administration and was supported by award no. MVP000. This publication does not represent the views of the VA, the US Food and Drug Administration, or the US Government. This research was also supported by funding from: the VA award I01-BX003362 (P.S.T. and K.-M.C.) and the VA Informatics and Computing Infrastructure (VINCI) VA HSR RES 130457 (S.L.D.). B.F.V. acknowledges support for this work from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (DK101478), the NIH National Human Genome Research Institute (HG010067) and a Linda Pechenik Montague Investigator award. K.-M.C., S.M.D., J.M.G., C.J.O., L.S.P., J.S.L., and P.S.T. are supported by the VA Cooperative Studies Program. S.M.D. is supported by the Veterans Administration [IK2-CX001780]. D.K. is supported by the National Heart, Lung, and Blood Institute of the NIH (T32 HL007734). K.H.K. is supported by NIH award UC4-DK-112217. K.S. is supported by NIH R01 DK087635. L.S.P. is supported in part by VA awards I01-CX001025, and I01CX001737, NIH awards R21DK099716, U01 DK091958, U01 DK098246, P30DK111024 and R03AI133172, and a Cystic Fibrosis Foundation award PHILLI12A0. We thank all study participants for their contribution. Data on T2D were contributed by investigators from the DIAMANTE Consortium, Biobank Japan, Malmö Diet and Cancer Study, PennCath, MedStar, Pakistan Genomic Resource, Penn Medicine Biobank, and Regeneron Genetics Center. Data on stroke were provided by MEGASTROKE investigators, and data on CKD were contributed by CKDgen investigators. Data on islet α- and β-cells were contributed by the HPAP Consortium (RRID:SCR_016202 and https://hpap.pmacs.upenn.edu/). Data on coronary artery disease were contributed by the CARDIoGRAMplusC4D investigators. We thank Josep Maria Mercader and Aaron Leong for careful review and comments.
Author information
Authors and Affiliations
Consortia
Contributions
M.V., J.M.K., K.-M.C., D.S., B.F.V., P.S.T. and C.J.O. were responsible for the concept and design. The acquisition, analysis or interpretation of data were performed by M.V., J.M.K., K.-M.C., D.S., B.F.V., P.S.T., R.L.J., C.T., T.L.A., J.E.H., J.Z., J.H., K.L., X.Z., J.A.L., A.T.H., K.M.L, D.K., S.P., J.D., O.M., A.R., N.H.M., S.H., I.H.Q., M.N.A., U.M., A.J., S.A., X.S., L.G., K.H.K., K.S., Y.V.S., S.L.D., K.C., J.S.L., J.M.G., L.S.P., D.R.M., J.B.M., P.D.R., P.W.W., T.L.E., D.J.R., S.M.D. and C.J.O. The authors M.V. and D.S. drafted the manuscript. The critical revision of the manuscript for important intellectual content was carried out by M.V., J.M.K., K.-M.C., D.S., B.F.V., J.A.L., P.S.T., C.T., J.Z., J.H., X.Z., D.K., X.S., L.G., K.H.K., K.S., L.S.P., J.B.M., P.D.R., T.L.E., S.M.D. and C.J.O. Finally, K.-M.C., D.S., and B.F.V. provided administrative, technical or material support.
Corresponding authors
Ethics declarations
Competing interests
None of the sponsors of the following authors had a role in the design and conduct of the study, in the collection, management, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript. D.S. has received support from the British Heart Foundation, Pfizer, Regeneron, Genentech and Eli Lilly pharmaceuticals. L.S.P. has served on Scientific Advisory Boards for Janssen, and received research support from Abbvie, Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, Abbvie, Vascular Pharmaceuticals, Janssen, Glaxo SmithKline, Pfizer, Kowa and the Cystic Fibrosis Foundation. L.S.P. is a cofounder, officer, board member and stockholder of the diabetes management-related software company Diasyst. S.L.D. has received research grant support from the following for-profit companies through the University of Utah or the Western Institute for Biomedical Research (an affiliated non-profit of VA Salt Lake City Health Care System): AbbVie, Anolinx, Astellas Pharma, AstraZeneca Pharmaceuticals, Boehringer Ingelheim International, Celgene Corporation, Eli Lilly and Company, Genentech, Genomic Health, Gilead Sciences, GlaxoSmithKline, Innocrin Pharmaceuticals, Janssen Pharmaceuticals, Kantar Health, Myriad Genetic Laboratories, Novartis International and PAREXEL International Corporation. P.D.R. has received research grant support from the following for-profit companies: Bristol Myers Squib and Lysulin, and has consulted with Intercept Pharmaceuticals and Boston Heart Diagnostics. S.M.D. receives research support to the University of Pennsylvania from RenalytixAI and consults for Calico Labs.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Trans-ethnic and ancestry-specific GWAS Manhattan plots.
a–d, Each graph represents a Manhattan plot. The y-axis corresponds to –log10 (P) for association with T2D in the respective ancestral group (a, Europeans (148,726 T2D cases, 965,732 controls, λ = 1.21); b, African American (24,646 T2D cases, 31,446 controls, λ = 1.08); c, Hispanics (8,616 T2D cases, 11,829 controls, λ = 1.03); d, Asians (46,511 T2D cases, 169,776 controls, λ = 1.15)). The x-axis represents chromosomal position on the autosomal genome. The y-axis truncated at 1 × 10−300. Points that are color-coded blue correspond to a P-value between 5.0 × 10−8 and 1.0 × 10−6. Points color-coded red indicate genome-wide significance (P = 5.0 × 10−8).
Extended Data Fig. 2 Trans-ethnic and ancestry-specific chromosome X Manhattan plots.
a–d, Each graph represents a Manhattan plot. The y-axis corresponds to –log10 (P) for association with T2D in the respective ancestral group (a, Europeans (69,869 T2D cases, 127,197 controls); b, African American (23,305 T2D cases, 30,140 controls); c, Hispanics (8,616 T2D cases, 11,829 controls); d, Asians (893 T2D cases, 1,560 controls)). The x-axis represents chromosomal position on chromosome X. The blue line corresponds with a significance threshold of P = 5.0 × 10−8. The red line corresponds with genome-wide significance (P = 5.0 × 10−8).
Extended Data Fig. 3 Results from PrediXcan analysis using GTEX data.
This graph represents an inverted Manhattan plot based on the output from the European T2D GWAS (148,726 T2D cases, 965,732 controls). The y-axis corresponds to –log10 (P) for association with genetically predicted gene expression in the respective tissue type (color coding shown on the right). Data were analyzed using S-PrediXcan software. The x-axis represents chromosomal position on the autosomal genome.
Extended Data Fig. 4 Manhattan plots for T2D-related complications using interaction analysis in individuals of European ancestry.
a–f, Each graph represents a Manhattan plot. The y-axis corresponds to –log10 (P) for association of SNP×T2D on T2D-related vascular outcome (a, coronary heart disease (56,285 cases, 140,945 controls, λ = 1.06); b, chronic kidney disease (67,403 cases, 129,827 controls, λ = 1.02); c, neuropathy (40,475 cases, 110,331 controls, λ = 1.03); d, peripheral artery disease (5,882 cases, 161,348 controls, λ = 1.02); e, retinopathy (13,881 cases, 123,538 controls, λ = 1.02); f, acute ischemic stroke (11,796 cases, 178,481 controls, λ = 1.00)). The x-axis represents chromosomal position on the autosomal genome. Points that are color-coded blue correspond to a P-value between 5.0 × 10−8 and 1.0 × 10−6. Points color-coded red indicate genome-wide significance (P = 5.0 × 10−8).
Extended Data Fig. 5 T2D PRS and the risk of T2D.
A shape plot representing the risk of a T2D genome-wide PRS (gPRS) on the odds ratio of T2D in MVP participants of European ancestry (69,869 T2D cases, 127,197 controls). The weights for the PRS have been obtained from an external reference dataset, namely the DIAMANTE Consortium. The gPRS has been divided into 10 deciles based on gPRS values in MVP white participants without T2D. The reference group is the lowest decile (0-10%). Odds ratios are shown as red dots, with their respective 95th percent confidence intervals displayed as red vertical lines.
Supplementary information
Supplementary Information
Supplementary Note
Supplementary Tables 1–26
Supplementary Tables 1–26
Source data
Source Data Fig. 2
Raw odds ratios for T2D-related outcomes shape plots
Source Data Extended Data Fig. 3
Raw effect estimates and P values for inverted Manhattan plot depicting genetically predicted gene expression using S-PrediXcan
Source Data Extended Data Fig. 5
Raw odds ratios for T2D shape plot
Rights and permissions
About this article
Cite this article
Vujkovic, M., Keaton, J.M., Lynch, J.A. et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 52, 680–691 (2020). https://doi.org/10.1038/s41588-020-0637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-020-0637-y
This article is cited by
-
Maternal smoking, nutritional factors at different life stage, and the risk of incident type 2 diabetes: a prospective study of the UK Biobank
BMC Medicine (2024)
-
Additive interaction of family medical history of diabetes with hypertension on the diagnosis of diabetes among older adults in India: longitudinal ageing study in India
BMC Public Health (2024)
-
Prevalence of chronic kidney disease in Tunisian diabetics: the TUN-CKDD survey
BMC Nephrology (2024)
-
Mapping the functional impact of non-coding regulatory elements in primary T cells through single-cell CRISPR screens
Genome Biology (2024)
-
Multi-ancestry polygenic mechanisms of type 2 diabetes
Nature Medicine (2024)