Abstract
Upon assembling the first Gossypium herbaceum (A1) genome and substantially improving the existing Gossypium arboreum (A2) and Gossypium hirsutum ((AD)1) genomes, we showed that all existing A-genomes may have originated from a common ancestor, referred to here as A0, which was more phylogenetically related to A1 than A2. Further, allotetraploid formation was shown to have preceded the speciation of A1 and A2. Both A-genomes evolved independently, with no ancestor–progeny relationship. Gaussian probability density function analysis indicates that several long-terminal-repeat bursts that occurred from 5.7 million years ago to less than 0.61 million years ago contributed compellingly to A-genome size expansion, speciation and evolution. Abundant species-specific structural variations in genic regions changed the expression of many important genes, which may have led to fiber cell improvement in (AD)1. Our findings resolve existing controversial concepts surrounding A-genome origins and provide valuable genomic resources for cotton genetic improvement.
Similar content being viewed by others
Main
Cultivated cotton is one of the most economically important crop plants in the world. The allotetraploid Upland cotton, G. hirsutum (n = 2x = 26, (AD)1), currently dominates the world’s cotton commerce1,2. Hybridization between the Old World A-genome progenitor and a New World D-genome ancestor, followed by chromosome doubling, formed the allopolyploid cotton ~1−2 million years ago (Ma)3,4. Uncertainty regarding the actual A-genome donor of the widely cultivated allotetraploid cotton G. hirsutum has persisted5,6,7,8,9,10,11,12,13. A1 (n = x = 13) and A2 (n = x = 13), commonly known as African and Asiatic cotton, respectively, are the only two extant diploid A-genome species in the world14. Stephens first proposed in Nature, using genetic and morphological evidence, that A2 was the A-genome donor of present-day allopolyploid cottons6. Gerstel argued via cytogenetic studies that A1 was more closely related to the A-genome in the allopolyploids than A2 (ref. 8). Despite recent efforts to sequence the cotton genomes, including Gossypium raimondii (D5)15,16, A2 (refs. 17,18), (AD)1 (refs. 10,19,20,21) and Gossypium barbadense10,21 ((AD)2, a much less cultivated tetraploid cotton), the origin history of the A-genome donor for the tetraploid (AD)1-genome5,11,13 and the extent of divergence between the A-genomes remain elusive22,23. Abundant studies support a Gossypium species resembling D5 as the D-genome donor13, but currently there is no solid evidence to suggest that the actual A-genome donor of tetraploid cottons is either A2 (refs. 6,7,10,19) or A1 (refs. 8,9,11,12,13) as has been suggested.
In this study, we assembled A1 variety africanum for the first time and re-assembled high-quality A2 cultivar Shixiya1 and (AD)1 genetic standard Texas Marker-1 (TM-1) genomes on the basis of PacBio long reads, paired-end sequencing and high-throughput chromosome conformation capture (Hi-C) technologies. Upon assembling and updating cotton genomes, we revealed the origin of cotton A-genomes, the occurrence of several transposable element (TE) bursts and the genetic divergence of diploid A-genomes worldwide. Also, we identified abundant structural variations (SVs) that have affected the expression of neighboring genes and help explain phenotypic differences among the cotton species.
Results
Sequencing and assembly of three high-quality cotton genomes
Here we sequenced the A1-genome var. africanum for the first time by generating ~225-gigabase (Gb) PacBio single-molecule real-time (SMRT) long reads (the N50 (minimum length to cover 50% of the total length) of these reads was 13 kilobases (kb)) with 138-fold genome coverage. We generated an assembly that captured 1,556 megabases (Mb) of genome sequences, consisting of 1,781 contigs with the N50 of these contigs reaching up to 1,915 kb (Table 1). The initial assemblies were then corrected by using highly accurate Illumina paired-end reads (Supplementary Table 1). Finally, 95.69% of total contigs spanning 1,489 Mb were categorized and ordered into 13 chromosome-scale scaffolds using Hi-C data (Table 1 and Supplementary Table 1).
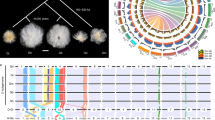
Also, the A2-genome cultivar Shixiya1 and the (AD)1-genome accession TM-1 were further sequenced using high-depth SMRT long reads resulting in 177-fold A2-genome coverage (~310 Gb) and 81.6-fold (AD)1-genome coverage (~205 Gb), respectively (Supplementary Table 1). The total assembled genome size for A2 was 1,637 Mb with 92.18% (1,509 Mb) of all sequences oriented and organized into 13 chromosomes. The resulting assembled genome size for (AD)1 was 2,290 Mb with 99.17% of all sequences anchored on 26 chromosomes (At1, 1,449 Mb; Dt1, 822 Mb). Compared with a recent PacBio-based A2 assembly18 (8,223 contigs with an N50 of 1,100 kb), our assembly consists of 2,432 contigs with N50 of 1,832 kb, resulting in a reduced number of gaps from 3,707 to 1,163 (Table 1 and Fig. 1a). The N50 of our updated (AD)1-genome is 5,020 kb (1,892 kb reported in ref. 21), with significantly fewer gaps compared with the most recently published genome (893 gaps versus 2,564 gaps reported in ref. 21), which represents ~2.65-fold improvement (Table 1 and Fig. 1b). Our assembled cotton genomes showed high congruence because the strongest signals from the Hi-C data clustered at the expected diagonal (Extended Data Fig. 1). Collinear relationships existed in quantity among cotton genomes, indicating that our pseudo-chromosomes derived from anchored and oriented contigs are of high quality (Extended Data Fig. 2). Our (AD)1-genome assembly also shared a high consistency for each chromosome with the previously published genetic map24 (Pearson correlation coefficients > 0.98) (Extended Data Figs. 3 and 4). These updated A2- and (AD)1-genomes may supplant earlier assemblies as chromosome-scale references.
a,b, Statistics of gap numbers in the assembly of A2- (a) and (AD)1- (b) genomes. A2*, previously released A2-genome18; At1* and Dt1* represent the At1- and Dt1-subgenome, respectively, of recently released (AD)1-genome21. c, Multi-dimensional display of genomic components of A1- and A2-genomes. The density was calculated per 1 Mb. I, the 13 chromosomes; II, gene density; III−V, coverage by TE, Gypsy and Copia, respectively; VI−VIII, transcriptional state in the ovule at 10 DPA and in root and leaf tissue, respectively. Transcript levels were estimated based on the average depth of mapped RNA reads in nonoverlapping 1-Mb windows. IX, GC content. d, Characterization of genomic variations in Gossypium. Genic synteny blocks are connected by gray lines. Reciprocal translocations and two large inversions are highlighted by dark gray and red links, respectively. e, Synteny maps using whole-genome alignments show that the inversion in chromosome 10 exists in either A1 or At1, whereas the one in chromosome 12 is found only in A1. Genomic homologous blocks ≥ 20 kb are drawn in the plots. Chr, chromosome.
The A1-, A2- and (AD)1-genomes comprise 43,952, 43,278 and 74,350 annotated protein-coding genes (Supplementary Table 2), respectively, mainly in both ends of the chromosomes because as much as 79.71% of A1, 80.06% of A2 and 64.09% of the (AD)1-genome are composed of TEs (Supplementary Table 3 and Fig. 1c). Also, TE-rich regions in the middle region of chromosomes remain silent, with low transcript levels, in contrast to gene-rich regions at both ends of chromosomes with high transcript levels (Fig. 1c).
Chromosomal translocation and inversions within Gossypium lineage
Compared with that of A1, the genome of A2 underwent a reciprocal translocation between chromosomes 1 and 2 (Fig. 1d), which is supported by previous cytogenetic data8. This translocation likely occurred after the two species separated and then became fixed in A2. The A1- and A2-genomes differed from the At1-subgenome by two and three translocations, respectively, of which the two reciprocal translocations between chromosomes 2 and 3, and 4 and 5, specifically occurred in the tetraploid At1-subgenome (Fig. 1d), suggesting that these translocations probably occurred after polyploidization. The two translocations that specially occurred in At1 were also confirmed by multivalent formations in hybrids between the allotetraploids and A1 or A2 (ref. 25). Two large-scale inversion events were detected between A1- and A2-genomes in chromosomes 10 and 12 that were confirmed by Hi-C data and also by PCR amplifications (Extended Data Fig. 5). The collinear relationship analysis of these cotton genomes indicated that the inversion in chromosome 12 specifically occurred in A1 with the syntenic blocks inverted at the diagonal between ~15.96 Mb and ~77.61 Mb; the inversion in chromosome 10 may have occurred either in A1 at the diagonal between ~18.4 Mb and ~61.3 Mb or in At1 between ~23.09 Mb and ~97.42 Mb (Fig. 1e). Thus, the two inversions likely occurred after the speciation of A1 and A2 cottons.
Origin of allotetraploid cotton
A molecular tree based on single-copy genes suggests that the common ancestor of the A1 and A2 clade was phylogenetically a sister to the At-subgenomes (At1 and At2) of (AD)1 and of (AD)2, respectively, and the divergence time for A1 and A2 was estimated to be ~0.7 Ma (0.4−1.4 Ma), well after the allotetraploid formation ~1.0−1.6 Ma (the values for the separation of At to A1 or A2, and Dt to D5) (Fig. 2a). Gene trees with specific recombination regions also supported the sister relationships between the A1–A2 clade and At1 (Extended Data Fig. 6a,b). Whole-genome phylogenetic analysis showed that the major topology 1 (At1, 56.17%; At2, 59.75%) supported the constructed species tree in Fig. 2a. The minor topology 2 with the sister relationship of A1 and At (At1, 22.22%; At2, 22.11%) had a slightly higher rate than the other minor topology 3 with the sister relationship of A2 and At (At1, 21.61%; At2, 18.14%) (Fig. 2b and Extended Data Fig. 6c). Synonymous substitution (Ks) analysis indicated that A1 and A2 had the lowest divergence (Ks values), compared with all other pairs (Fig. 2c). Likewise, a significantly greater number of identical sites were found between orthologs of A1 versus A2 relative to either A1 or A2 versus At1 or At2 (Fig. 2d). We further selected representative cotton lines, including 30 (AD)1, 14 A1 and 21 A2 accessions, to construct a phylogenetic tree based on whole-genome SNP studies to further validate the relationships of A1, A2 and At1 (Fig. 2e and Extended Data Fig. 7). Because the actual A-genome donor may be extinct, we compared At1, A1 and A2 accessions with the D5, an outgroup for all A-genome species. The distance from D5 to At1 was much smaller than that from D5 to its previously thought common ancestor, A1 or A2. About 30.54% of the SNPs of At1 were identical to the corresponding sites in the D5-genome, whereas only 20.52% and 20.04% of ancestral alleles of A1 and A2, respectively, were identical to the corresponding sites in D5-genome (Fig. 2e). The nucleotide variation analysis indicated that A1 has relatively fewer nucleotide variations than A2 compared with At1 across the 13 chromosomes (Fig. 2f). Based on these evidence, we constructed a revised model in which neither A1 nor A2 is the actual A-genome donor. Instead, hybridization between the common ancestor (A0) of all A-genomes (A1, A2 and At) and a D5-genome resembling G. raimondii formed the allotetraploid cotton (Fig. 2g). Our results also indicated that the A0, inferred as the possible At donor, was more phylogenetically related to A1 than A2. The AD ((AD)1 and (AD)2) tetraploidization occurred approximately 1.0−1.6 Ma; A0 then developed into two A-genomes around 0.7 Ma (Fig. 2h). The finding that A0 is a common ancestor for A1, A2, the At1-subgenome in (AD)1 and the At2-subgenome in (AD)2 resolves a puzzle regarding previous inconsistent phylogenetic data6,7,8,9,11,12,13 and explains why interspecific hybridization of A1 or A2 with D5 is often unsuccessful, because the genetic distances between the current A- and D-genomes are great enough to preclude fertilization.
a, Inferred phylogenetic analysis among Gossypium and other eudicot plants. b, Summary of phylogenetic analysis with the approximately unbiased test in 10-kb windows. c, Distribution of Ks values for orthologous genes among cotton genomes. Peak values for each comparison are indicated in the parentheses. d, Comparisons of identical sites in orthologous genes. Violin plots summarize the distribution of identical sites. The center line in each box indicates the median, and the box limits indicate the upper and lower quartiles of divergence (n = 20 types of synonymous mutation). P values were derived with Student’s t-test. e, Phylogenetic and ancestral allele analysis based on SNPs. The red, blue and green triangles represent the collapsed 21 A2 accessions, 14 A1 accessions and 30 (AD)1 accessions, respectively. The percentage value indicates the percentage of ancestral alleles for each species that were identical to those of the D5-genome. f, Number of nucleotide variations in A1 or A2 compared with At1 across the chromosomes. g, A model for the formation of allotetraploid cotton showing fiber phenotypes from the (AD)1 (accession TM-1), the D5, the A1 (var. africanum) and the A2 (cv. Shixiya1). Scale bar, 5 mm. h, A schematic map of the evolution of cotton genomes. Major evolutionary events are shown in dashed boxes.
Population genomic study of two A-genome species
We collected 14 A1 and 67 A2 representative cotton accessions from India, Pakistan, China and other countries to study the genetic divergence between A1 and A2 (Fig. 3a and Supplementary Table 4). All resequencing reads with an average coverage depth of ~7.2× for each accession were mapped to our assembled A2-genome for SNP identification. A total of 11,652,404 SNPs and 1,716,908 indels (ranging from 1 to 259 base pairs (bp) in length) were identified (Supplementary Table 5). Principal component analysis (PCA) based on SNPs showed that, despite their geographic origins, these cotton accessions were clustered in two independent groups: the A1 group and A2 group (Fig. 3b). The neighbor-joining tree using SNPs indicated that A1 and A2 clustered in two independent clades, and A2 from India and Pakistan and A2 from China have the closest relatives (Fig. 3c), which was confirmed by sliding window phylogenetic analysis with an average weighting of 55% in topology 1 (Fig. 3d). The topology 2 is nearly identical to topology 3 throughout chromosomes, but there are several weak shifts in support toward topology 2 potentially reflecting the introgression between A1 and A2 distributed in China (Fig. 3e and Extended Data Fig. 8). Model-based clustering showed that the population structures of A1 accessions were obviously significantly different from A2 accessions (number of clusters (K) = 2), and the population divergence between the A1 and A2 from the India and Pakistan group or from China reached almost 1.0, which suggested that these differences clearly distinguish A2 from A1 as two cotton species, and may explain the phenomenon in which interspecific hybridizations of A2 with A1 are often unsuccessful (Fig. 3c,f). Several A2 accessions from India and Pakistan were clustered sisterly to all A2 accessions collected from China, and the accessions from China had distinct population structures from accessions from India and Pakistan (K = 3). According to our results and the recorded history of Chinese Asian cotton26, we concluded that A2 was likely introduced to China from India and/or Pakistan, then developed into a distinct geographical race (Fig. 3c). Two accessions of A1 var. africanum were gathered at the root of all other A1 accessions with no obvious impact on A2 development, which did not support the notion that africanum is the source of both cultivated A1- and A2-genomes14 (Fig. 3g). The large genetic differences revealed by population analysis and chromosomal SVs between A1 and A2 suggest that two A-genomes were evolved independently, with A1 var. africanum as the only living ancestor of A1 accessions.
a, Geographic distribution of the collected A1 and A2 accessions. Green, red and yellow dots represent A1 accessions and A2 accessions collected in China and outside of China, respectively. The map was drawn using the maptools package (http://maptools.r-forge.r-project.org/). b, PCA plots of the first three components for A1 and A2 accessions. Dot colors are the same as in a. c, Analysis of genetic relationship between all A1 and A2 accessions. The upper and lower panels show the phylogenetic tree based on whole-genome SNP studies and population structure of all accessions based on different numbers of clusters (K = 2–3), respectively. Branch colors are the same as in a. CHG, A2 accessions from the China group; IPG, A2 accessions from the India and Pakistan group. d, Average weightings for the three possible topologies in whole genomes. e, Weightings for all three topologies described in d across chromosome 7 using sliding windows. f, Population divergence (FST) across the three groups described in c. g, Phylogenetic analysis based on SNPs. The yellow and green triangles represent the collapsed 67 A2 accessions and 12 A1 accessions, respectively. Two A1 var. africanum accessions (Ghe01 and Ghe04) gathered at the root of the 12 A1 accessions. PC1, the first principal component (PC); PC2, the second PC; PC3, the third PC.
Genome expansions and evolution
Among genome-sequenced plants of the order Malvales27,28,29,30, D5 and the Dt1-subgenome in (AD)1 are similar in genome sizes relative to Bombax ceiba or Durio zibethinus, but are expanded nearly twofold compared with the Theobroma cacao and Corchorus capsularis genomes (Fig. 4a). The two A-genomes and the At1-subgenome experienced a further twofold expansion that was highly correlated with TE bursts (correlation coefficient, R2 = 0.978) (Fig. 4a). While both the D5-genome (738 Mb) and Dt1-subgenome (822 Mb) are nearly equivalent in size relative to the D. zibethinus genome (715 Mb), long terminal repeat (LTR) families in Gossypium (52.42% of the Dt1-subgenome, 53.2% of the D5-genome) were greatly expanded in comparison to D. zibethinus (26.2%). As much as 72.57% of the A1-genome and 73.62% of the A2-genome were composed of LTRs (Fig. 4b). LTR retrotransposons in Gossypium and B. ceiba have experienced continuing and more recent amplification bursts from 0−2 Ma, while D. zibethinus underwent a distinct amplification burst event around 8−10 Ma (Fig. 4c). LTR retrotransposons in the A2-genome were further classified into 64 families, of which 68% belonged to the Gypsy superfamily and 12.6% to Copia (Fig. 4d). By using representative LTR/Gypsy sequences (Supplementary Fig. 1) to evaluate TE hits in cotton genomes, five distinct insertion peaks for the Gypsy-type LTR with identities from 65−76% to 96.4−99.4% were observed in different cotton genomes (Fig. 4e). We used our Gaussian probability density function (GPDF) analysis to estimate the burst time of major peaks, finding that the earliest insertion event occurred ~5.7 Ma, which is the expected speciation time for A- and D-genomes (Extended Data Fig. 9 and Fig. 4f). The peak with 85.5−88.5% identity, corresponding to ~2.0 Ma, is found specifically in Dt1- and At1-genomes, but not in D5, A1 or A2, suggesting that the allotetraploid cotton may have formed as early as ~2.0 Ma. The peak with 87−89.5% identity corresponded to 0.89 Ma and is common to both A1 and A2, indicating that speciation might occur at a later time. Indeed, the 93.0−93.8% identity (or 0.61 Ma) peak is unique to A1, and the last peak (with 96.4−99.4% identity; no valid calculation of ages because it is too close to date) is A2-specific. Our data showed that A1 and A2 speciation occurred 0.89−0.61 Ma. This was confirmed by results (Supplementary Fig. 2a–c, TDIV1 (divergence time between A1 and A2) = 1,016,499 yr) obtained from fastsimocoal2 analysis, which used 30 accessions from (AD)1, 14 from A1 and 21 from A2, as reported in Fig. 2e. However, G-PhoCS analysis, which used data from the fully assembled A1-, A2- and (AD)1-genomes (Supplementary Fig. 2d–f), did not quite fit our previous model. We suggest that G-PhoCS may not fit well for evolutionary analysis of genomes with high TE contents, such as cotton.
a, Genome size expansion is highly correlated with TE amplification bursts (R2 = 0.978). The red line shows the linear relationship between genome size and TE content. b, Genomic component comparisons among genome-sequenced Malvales plants. c, Analysis of intact LTR numbers and insertion time in Malvales plants. d, Classification of intact LTRs in the A2-genome. LTR families with a copy number of ≥100 are shown. e, Identity distribution pattern of TE hits presented as a dot-plot. The most recent LTR/Gypsy sequence of LTR families was selected as the representative sequence for detecting additional TE hits in the genomes. A total of 262,377 dots in D5, 585,658 in Dt1, 3,541,372 in At1, 4,218,810 in A1 and 5,035,006 in A2 were drawn in the dot-plot. P1–P5 represent the identified five distinct bursts in different cotton genomes. f, Number of TE hits for the representative sequence and their associated identity values. The estimated burst time based on GPDF fitting of each peak is marked. The five peaks, P1–P5, defined in e are highlighted by shaded gray columns. LINE, long interspersed nuclear elements; SINE, short interspersed nuclear elements.
SVs and fiber development
SVs including large deletions and insertions (>50 bp) are reported to drive important phenotypic variation within species31. Here we found that (AD)1 fiber cells underwent fast elongation reaching up to 30.5 ± 0.7 mm until 30 d post anthesis (DPA), whereas fiber cells in A1 (14.7 ± 0.7 mm) and A2 (16.1 ± 0.9 mm) elongated at a slower rate and terminated earlier (~20 DPA) (Fig. 5a). By comparing two A-genomes with the A-subgenome of (AD)1, we identified 39,476 deletion and 21,577 insertion events in A1, as well as 40,480 deletion and 20,903 insertion events in A2. Meanwhile, we obtained 35,997 common SVs events including 21,431 deletions and 14,566 insertions in A1 and A2, suggesting that these SVs occurred mainly at the common ancestor stage of two A-genome species (Fig. 5b). Of the total common SVs, 11,395 events (31.66%) were overlapped with genic regions affecting 9,839 unique genes, with 912 events occurring in coding DNA sequences (CDSs), 1,105 in introns and 9,378 in up-/downstream regions (Fig. 5c and Supplementary Table 6). Of the reported 1,753 associated loci for fiber traits2,32, 460 associated loci contained common SVs, with those in up-/downstream regions as the major type (Supplementary Table 7). We identified 1,545 upregulated and 1,908 downregulated genes by comparing transcriptomes of rapidly elongating fiber cells from the At1-subgenome with those of A2 (Supplementary Table 8). Also, 2,941 upregulated and 3,350 downregulated genes were identified with At1 and A1 comparisons at elongating fibers (Supplementary Table 9). Of these differentially expressed genes, 949 for At1 versus A2 and 1,687 for At1 versus A1 contained common SVs, respectively (Fig. 5d, Extended Data Fig. 10 and Supplementary Tables 10 and 11). Gene ontology enrichment analysis indicated that fatty acid biosynthesis, cell wall deposition or biogenesis, and carbohydrate metabolism were the most enriched biological processes (Fig. 5e). Quantitative PCR with reverse transcription (RT–qPCR) analysis of several key genes related to fatty acid biosynthesis, including encoding 3-ketoacyl-CoA synthase (KCS), fatty acid hydroxylase (WAX2) and lipid transport proteins, validated the upregulation pattern in At1 compared with both A1 and A2 (Fig. 5f,g). Large sequence variations existed between At1 and A1 or At1 and A2 in the upstream or downstream regions of all of these genes (Supplementary Fig. 3). We introduced KCS6, a key gene in very-long-chain fatty acid biosynthesis33,34, in G. hirsutum cv. Zhong24 background and observed a significant increase (~6.0–11.66%) of final fiber lengths in three homozygous transgenic lines (L241-1, L241-2, L241-3) that were driven by 35S promoter and one line (L245-1) driven by the fiber-specific E6 promoter (Fig. 5h). Fifty-six transcription factors, including WRKY12, HD-Zip2 and MYB6, showed differential expression patterns among the three cotton species that can be correlated with SVs (Fig. 5i and Supplementary Table 12). In combination with genome scanning of transcription factor binding sites and A2–At1 differential expression, we identified 198 potential target genes for WRKY12 and 232 for HD-Zip2 in the cotton genome (Supplementary Tables 13 and 14). We suggest that higher expression intensities of these potential target genes in (AD)1 may lead to longer fibers in (AD)1 than in either A1 or A2.
a, Comparisons of fiber elongation patterns. The center line in each box indicates the median, and the box limits indicate the upper and lower quartiles (n = 30 seeds). b, SVs of two A-genomes compared with the At1-subgenome. c, Annotation of identified common SVs in genic regions. Up-/downstream, 5 kb regions from the start or stop codons. d, Volcano plots for A2~At1 gene expression in elongating fibers at 15 DPA. Each hollow point represents a gene and genes with SVs within 5 kb of their start or stop codons are indicated by a triangle. Dashed lines show the thresholds (P ≤ 0.001 and twofold change between A2 and At1). e, Gene ontology enrichment of significant differentially expressed genes with SVs (P ≤ 0.01). f, Upregulated genes in fatty acid biosynthetic process. Red items, upregulated genes in At1 relative to A2 at 15 DPA. g, RT–qPCR analysis of upregulated genes in fatty acid biosynthetic pathway in elongating fibers at 5–20 DPA. UBQ7 was used as a normalization control (mean ± s.d, n = 3 independent experiments). h, Cotton fibers of the WT (G. hirsutum cv. Zhong24) and the transgenic lines expressing KCS6 gene under control of the CaMV 35S promoter (L241-1, L241-2 and L241-3) or E6 promoter (L245-1). The averaged fiber lengths with standard errors are denoted under each cotton line using Student’s t-test. Scale bar, 5 mm. i, RT–qPCR analysis of three upregulated potential transcription factor genes in elongating fibers at 5–20 DPA (mean ± s.d., n = 3 independent experiments). WT, wild type.
Discussion
With high-quality assembly of two African–Asian species, A1 and A2, we provided a more complete landscape of genome architecture, gene annotations and TE insertions, which is critical to evolutionary and comparative genomics as well as genetic variation analysis. Our data suggested that At may have originated from a primitive A-genome common ancestor, referred to here as A0, instead of extant A1 or A2. Allotetraploid formation preceded the speciation of the present two A-genomes, and then A1 and A2 originated independently with no ancestor–progeny relations. Upon publication of our new data, we anticipate that reviews and textbooks7,11,35 related to cotton genome evolution will have to be revisited and revised.
Several LTR bursts contributed compellingly to A-genome size expansion, speciation and evolution. By using fragmented coding sequences of LTRs, our GPDF analysis overcame a major pitfall related to most previous similar studies that relied on the presence of both ends of full-length LTRs10,17,36,37, such that more recently inserted LTRs are likely over-represented. We suggest that GPDF may be applied to analyze accurately the time of LTR bursts and genome evolution. Analysis of SV and gene expression patterns identified putative candidates to investigate the phenotypic difference among three cotton species. These candidate genes would enable cotton breeders to further improve major agronomical traits such as fiber quality and yield.
Methods
Sampling and sequencing
Genomic DNA molecules of G. herbaceum (var. africanum Mutema, A1-0076), G. arboreum (cv. Shixiya1) and G. hirsutum (TM-1) were isolated from young leaves of individual plants. We obtained polymerase reads of ~225 Gb, ~177 Gb and ~205 Gb from SMRT cells on PacBio RSII and Sequel instruments for G. herbaceum (A1), G. arboreum (A2) and G. hirsutum (AD)1, respectively. Our previously released ~133-Gb PacBio reads from A2 were also integrated into our current A2-genome assembly. For A1-, A2- and (AD)1-genomes, we also obtained ~52 Gb, 95 Gb and 70 Gb of raw reads, respectively, with 400-bp inserts using a whole-genome shotgun approach on the Illumina HiSeq X-Ten platform. We sequenced ~256 Gb of clean Hi-C data for A1, ~219 Gb of clean Hi-C data for A2 and ~196 Gb of clean Hi-C data for (AD)1 on the Illumina HiSeq platform.
Assembly and correction
We performed de novo assembly of PacBio long reads into contigs with the program Falcon (v.0.4)38. To further improve the accuracy of reference assembled contigs, two-step polishing strategies were performed: we first used PacBio long reads and carried out an initial polishing with Polish software39 and then used highly accurate Illumina paired-end reads to further correct the assembly with Pilon (v.1.20) software40. The PacBio contigs were further clustered and extended into pseudo-chromosomes using Hi-C data. Gaps that existed in the genomes were filled using Pbjelly41, followed by a second round of polishing using Quiver39.
Repeat analysis
Each of the whole genomes was searched for repetitive sequences including tandem repeats and TEs. Tandem repeats were annotated by TRF (v.4.07b)42 with the following parameters: 2, 7, 7, 80, 10, 50, 2,000. TE annotations were identified using a combination of de novo and homology-based approaches. A de novo repeat library was constructed with RepeatModeler (v.1.0.8). We adapted RepeatMasker (v.4.0.6)43 to search for similar TEs against Repbase (Repbase21.08)44, mips-REdat library and the de novo repeat library. The RepeatProteinMask program was used to search against a TE protein database.
Analysis of potential LTR bursts using fragmented Gypsy-type transposons derived from full-length sequences
Intact LTR retrotransposons were detected using LTR_FINDER45 and classified into 64 families with 5′-LTR sequences based on the following parameters: similarity ≥ 80%, coverage ≥ 80% and copy number ≥ 100. A total of 13,332 LTR retrotransposons were translated in six frames that produced 1,397 Gypsy sequences with amino acids > 1,000.
GPDF fitting of LTR identity distributions and LTR burst time calculations
Full-length and truncated LTRs were identified across genomes with various lengths and identities, and then each sequence (length = l) was divided into 30-bp units to determine the number of dots (n = l/30) with the same identity. Each Gypsy superfamily sequence was normalized to dot arrays with various identities, and all dot arrays were used to generate a box-plot according to their identities. For GPDF fitting and burst time calculation, single peaks in the TEs identity distribution curves were separated and fitted by GPDF with high adjusted R2 values, and the average nucleotide substitution ratio (K) was defined as 2.58 standard deviations (σ). Then the TE burst time point for individual amplification peaks was estimated by t = K/r, in which r is the nucleotide substitution rate for cotton species (r = 7 × 10−9)17.
Gene prediction and annotation
Homology-based prediction, RNA-sequencing-assisted prediction and ab initio prediction were used for gene model prediction. For homology-based prediction, GeMoMa software46 was applied based on homologous proteins from sequenced species, which included Arabidopsis thaliana (TAIR10, http://www.arabidopsis.org/), Oryza sativa (v7.0), G. arboreum, G. hirsutum, G. raimondii (D5), Populus trichocarpa (v.3.1), T. cacao (http://cocoa-genome-hub.southgreen.fr) and Vitis vinifera (Genoscope 12×). RNA sequencing transcripts assembled with HISAT47 and StringTie48 were used to assist in gene structure predictions (Supplementary Table 15). In summary, a total of 52,444 (mean size: 2,177.9 bp), 56,130 (mean size: 2,414.7 bp) and 111,872 (mean size: 1,892.1 bp) assembled transcripts were obtained for A1, A2 and (AD)1, respectively. For ab initio gene prediction, we applied SNAP (V2006-07-28)49, Augustus (v.3.2.2)50, Genscan51 and GlimmerHMM (v.3.0.4)52 to generate gene structures. Finally, all predictions were integrated to produce a consensus gene set using EVidenceModeler (v.1.1.1)53. Gene functional annotations were assigned by aligning protein sequences to Swiss-Prot and TrEMB54 using BLASTP (E value (expected value) ≤ 1 × 10−5), KAAS55 (v.2.1) and InterProScan56 (v.5.24). Gene Ontology57 IDs for each gene were extracted from the InterPro entry.
Phylogenetic analysis
We used BLASTP to generate protein sequence pairs (E value ≤ 1 × 10−5) and then OrthoMCL (v.2.0.9)58 to cluster gene families with an inflation value of 1.5. The single-copy gene families were extracted and aligned using MAFFT (v.7.058)59. A phylogenetic tree was constructed using a maximum likelihood method implemented in RAxML (v.8.0.19)60 with a GTRGAMMA substitution model with O. sativa as the outgroup. The Markov chain Monte Carlo algorithm for Bayes estimation was adopted to calculate the divergence time using PAML (v.4.6)61. For the identification of SNPs on orthologous genes among A1, A2, At1 in (AD)1 and At2 in (AD)2 (ref. 21), we used BLASTP to do pairwise alignments and retained only homologous gene pairs with reciprocal best hits (E value ≤ 1 × 10−5). Then we generated multiple alignments of homologous proteins and back-translated to the CDS. A SNP was determined to be present if a position in the alignment included two or more different bases. If a SNP was identified in the aligned CDS but no resulting amino acid variation occurred in the corresponding position of alignment, this site was defined as an identical site within the ortholog. To further understand phylogenetic relationships among A1, A2 and At1, we focused on specific recombination regions to infer gene trees according to a previous report62. We applied reported methods63 to further perform phylogenetic analysis among A1, A2 and At1 or At2. In brief, genome alignments were divided into 10-kb segments and we performed an approximately unbiased test. The site likelihoods for each possible topology were calculated by RAXML, then these likelihoods were input into Consel64.
SNP identification
The sequenced reads of 14 A1 and 67 A2 cotton accessions were mapped to our assembled A2-genome in this study using BWA (0.7.10-r789)65. PCR duplications in the alignments were removed in Picard (v.1.94). SNPs and indels identified by the HaplotypeCaller module were then used to perform base-quality recalibration with the BaseRecalibrator and IndelRealigner modules in the GATK toolkit (v.3.8)66. The genomic variants in GVCF (genomic variant call format) for each accession as identified by the HaplotypeCaller module and the GVCF files were merged. Raw SNP calls were further filtered using GATK filter expressions (‘QUAL<30.0||QD<2.0||FS>60.0||MQ<40.0||SOR>4.0’ --clusterWindowSize 5 --clusterSize 2).
Population genetics analysis
A subset of 9,555,165 SNPs (max-missing > 0.5, minor allele frequency > 0.05) in the 14 A1 and 67 A2 cotton accessions was screened to build a neighbor-joining tree in MEGA7 (ref. 67) with 1,000 bootstrap replicates using D5 as the outgroup. The cotton population structure analysis and a PCA were carried out using admixture68 with K values from 2 to 3 and EIGENSOFT software69, respectively. A pairwise fixation statistic (FST) analysis as calculated in the PopGenome package70 was used to estimate the degree of variability in three groups (A1 accessions worldwide, A2 accessions from the India and Pakistan group, and A2 accessions from the China group). To validate the relationships of the A1, A2 and At1, we used 30 released (AD)1 accessions32,71, 21 released A2 accessions and 14 released A1 accessions to construct a population phylogenetic tree with D5 as the outgroup (Extended Data Fig. 7). The identification of ancestral alleles was as described18.
Phylogenetic weighting
For genome-wide evaluation of three possible phylogenetic hypotheses, a method called Twisst72,73 was applied to analyze A1 and A2 accessions. In brief, the phasing and imputation of filtered SNPs (minAlleles 2, depth (DP) ≥ 5, genotype quality (GQ) ≥ 30) obtained from the 14 A1 and 67 A2 accessions and the outgroup D5 were performed using Beagle software with default parameters. Trees were constructed for each sliding window of 50 SNPs across 13 chromosomes using Phyml software, then tree weightings were computed using Twisst, with four defined taxa: D5, A1 and A2 from China, and from India/Pakistan.
Demographic analysis
The G-PhoCS74 method was employed to infer the complete demographic history for A1, A2 and At1 based on 2,468 selected neutral loci. Coalescence simulations were run under two models, M1 (no gene flow) and M2 (ancient gene flow). To further convert estimates of divergence time (τ) and population size (θ) from mutations per site to years (T) and effective numbers of individuals (Ne), respectively, we assumed an A1A2–At1 average genomic divergence time of Tdiv ≈ 1.0 Ma (0.6–1.8 Ma), which was calculated by the molecular tree based on single-copy genes, and an annual production. We further applied fastsimcoal2 software75 to infer demographic history based on fourfold degenerate sites selected from SNP datasets (minor allele frequencies > 0.05) from 30 released (AD)1 accessions, 21 released A2 accessions and 14 released A1 accessions.
SVs among three cotton genomes
SVs were identified using NGMLR (v.0.2.4)76 and PbSV (v.0.1.0). First, we mapped the PacBio subreads of A1 and A2 to the genome of (AD)1 using NGMLR with default parameters, and then PbSV was used to find large indels with length >50 bp using parameters: gapdistance = 1,000, min_readcount = 2, min_readfraction = 0.2, positionwiggle = 200, basepairidwiggle = 0.25, call_min_mapq = 10.
RT–qPCR analysis and plant transformation
Total RNA (~2 μg) was extracted and was then reverse transcribed in a 20-μl reaction mixture with TransScript cDNA Synthesis SuperMix (TransGen Biotech). Then 1-μl sample aliquots were used as templates for RT–qPCR analysis. UBQ7 was used as the internal control for RT–qPCR data analysis. The CDS sequences of the KCS6 gene were PCR amplified from the complementary DNA of 10-DPA fiber tissue and cloned into the pCAMBIA2300 vector, forming 35S::KCS6 or E6::KCS6 constructs. Then the construct was introduced into Agrobacterium tumefaciens strain LBA4404, and subsequently transferred into the Upland cotton G. hirsutum cv. Zhong24. All primers used in this study are presented in Supplementary Table 16.
Statistical analyses
Student’s two-tailed t-tests were performed in GraphPad Prism software.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The genome sequence data for A1 and A2 are deposited in NCBI (PRJNA506494). The (AD)1-genome sequence data are accessible through NCBI (PRJNA524970). The assemblies and annotation files of A1, A2 and (AD)1 are available at the CottonGen website (https://www.cottongen.org/). The re-sequence data for A1 and A2 accessions can be accessed with accession number PRJNA507537 in NCBI. Source data for Figs. 2 and 5 and Extended Data Fig. 5 are presented with the paper.
References
Wu, Z. et al. Cotton functional genomics reveals global insight into genome evolution and fiber development. J. Genet. Genomics 44, 511–518 (2017).
Ma, Z. et al. Resequencing a core collection of upland cotton identifies genomic variation and loci influencing fiber quality and yield. Nat. Genet. 50, 803–813 (2018).
Senchina, D. S. et al. Rate variation among nuclear genes and the age of polyploidy in Gossypium. Mol. Biol. Evol. 20, 633–643 (2003).
Webber, J. M. Cytogenetic notes on cotton and cotton relatives. II. Science 84, 378 (1936).
Zahn, L. M. Unraveling the origin of cotton. Science 335, 1148 (2012).
Stephens, S. G. Phenogenetic evidence for the amphidiploid origin of New World cottons. Nature 153, 53–54 (1944).
Hutchinson, J. B., Silow, R. A. & Stephens, S. G. (eds) The Evolution of Gossypium and the Differentiation of the Cultivated Cottons (Oxford Univ. Press, 1947).
Gerstel, D. Chromosomal translocations in interspecific hybrids of the genus Gossypium. Evolution 7, 234–244 (1953).
Palmer, S. A. et al. Archaeogenomic evidence of punctuated genome evolution in Gossypium. Mol. Biol. Evol. 29, 2031–2038 (2012).
Hu, Y. et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 51, 739–748 (2019).
Wendel, J. F., Brubaker, C., Alvarez, I., Cronn, R. & Stewart, J. M. Genetics and Genomics of Cotton Vol. 3 (ed. Paterson, A. H.) Ch. 1 (Springer, 2009).
Endrizzi, J. E., Turcotte, E. L. & Kohel, R. J. Genetics, cytology, and evolution of Gossypium. Adv. Genet. 23, 271–375 (1985).
Wendel, J. F. New World tetraploid cottons contain Old World cytoplasm. Proc. Natl Acad. Sci. USA 86, 4132–4136 (1989).
Kulkarni, V. N., Khadi, B. M., Maralappanavar, M. S., Deshapande, L. A. & Narayanan, S. S. Genetics and Genomics of Cotton Vol. 3 (ed. Paterson, A. H.) Ch. 4 (Springer, 2009).
Wang, K. et al. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 44, 1098–1103 (2012).
Paterson, A. H. et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 492, 423–427 (2012).
Li, F. et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nat. Genet. 46, 567–572 (2014).
Du, X. et al. Resequencing of 243 diploid cotton accessions based on an updated A genome identifies the genetic basis of key agronomic traits. Nat. Genet. 50, 796–802 (2018).
Li, F. G. et al. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 33, 524–530 (2015).
Zhang, T. Z. et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 33, 531–537 (2015).
Wang, M. et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 51, 224–229 (2019).
Hutchinson, J. New evidence on the origin of the Old World cottons. Heredity 8, 225–241 (1954).
Renny-Byfield, S. et al. Independent domestication of two Old World cotton species. Genome Biol. Evol. 8, 1940–1947 (2016).
Wang, S. et al. Sequence-based ultra-dense genetic and physical maps reveal structural variations of allopolyploid cotton genomes. Genome Biol. 16, 108 (2015).
Menzel, M. Y. & Brown, M. S. The significance of multivalent formation in three-species Gossypium hybrids. Genetics 39, 546–557 (1954).
Watt, G. The Wild and Cultivated Cotton Plants of the World (Longmans, Green and Co., 1907).
Teh, B. T. et al. The draft genome of tropical fruit durian (Durio zibethinus). Nat. Genet. 49, 1633–1641 (2017).
Argout, X. et al. The genome of Theobroma cacao. Nat. Genet. 43, 101–108 (2011).
Islam, M. S. et al. Comparative genomics of two jute species and insight into fibre biogenesis. Nat. Plants 3, 16223 (2017).
Gao, Y. et al. De novo genome assembly of the red silk cotton tree (Bombax ceiba). GigaScience 7, giy051 (2018).
Sun, S. et al. Extensive intraspecific gene order and gene structural variations between Mo17 and other maize genomes. Nat. Genet. 50, 1289–1295 (2018).
Fang, L. et al. Genomic analyses in cotton identify signatures of selection and loci associated with fiber quality and yield traits. Nat. Genet. 49, 1089–1098 (2017).
Qin, Y. M. et al. Saturated very-long-chain fatty acids promote cotton fiber and Arabidopsis cell elongation by activating ethylene biosynthesis. Plant Cell 19, 3692–3704 (2007).
Xiao, G. H., Wang, K., Huang, G. & Zhu, Y. X. Genome-scale analysis of the cotton KCS gene family revealed a binary mode of action for gibberellin A regulated fiber growth. J. Integr. Plant Biol. 58, 577–589 (2015).
Wendel, J. F. et al. Polyploidy and Genome Evolution (eds Soltis, P. S. & Soltis, D. E.) Ch. 10 (Springer, 2012).
Ling, H. Q. et al. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 557, 424–428 (2018).
Banks, J. A. et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332, 960–963 (2011).
Chin, C. S. et al. Phased diploid genome assembly with single molecule real-time sequencing. Nat. Methods 13, 1050–1054 (2016).
Chin, C. S. et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 10, 563–569 (2013).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9, e112963 (2014).
English, A. C. et al. Mind the gap: upgrading genomes with Pacific Biosciences RS long-read sequencing technology. PLoS ONE 7, e47768 (2012).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27, 573–580 (1999).
Tarailo-Graovac, M. & Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinformatics 25, 4.10.1–4.10.14 (2009).
Kapitonov, V. V. & Jurka, J. A universal classification of eukaryotic transposable elements implemented in Repbase. Nat. Rev. Genet. 9, 411–412 (2008).
Xu, Z. & Wang, H. LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 35, W265–W268 (2007).
Keilwagen, J. et al. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 44, e89 (2016).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Korf, I. Gene finding in novel genomes. BMC Bioinformatic 5, 59 (2004).
Stanke, M. et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34, W435–W439 (2006).
Burge, C. & Karlin, S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 268, 78–94 (1997).
Majoros, W. H., Pertea, M. & Salzberg, S. L. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics 20, 2878–2879 (2004).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 9, R7 (2008).
Bateman, A. et al. UniProt: a hub for protein information. Nucleic Acids Res. 43, D204–D212 (2015).
Moriya, Y., Itoh, M., Okuda, S., Yoshizawa, A. C. & Kanehisa, M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 35, W182–W185 (2007).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Li, L., Stoeckert, C. J. Jr. & Roos, D. S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13, 2178–2189 (2003).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Yang, Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13, 555–556 (1997).
Pease, J. B. & Hahn, M. W. More accurate phylogenies inferred from low-recombination regions in the presence of incomplete lineage sorting. Evolution 67, 2376–2384 (2013).
Schumer, M., Cui, R., Powell, D. L., Rosenthal, G. G. & Andolfatto, P. Ancient hybridization and genomic stabilization in a swordtail fish. Mol. Ecol. 25, 2661–2679 (2016).
Shimodaira, H. & Hasegawa, M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17, 1246–1247 (2001).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Pfeifer, B., Wittelsbürger, U., Ramos-Onsins, S. E. & Lercher, M. J. PopGenome: an efficient Swiss army knife for population genomic analyses in R. Mol. Biol. Evol. 31, 1929–1936 (2014).
Wang, M. et al. Asymmetric subgenome selection and cis-regulatory divergence during cotton domestication. Nat. Genet. 49, 579–587 (2017).
Martin, S. H. & Van Belleghem, S. M. Exploring evolutionary relationships across the genome using topology weighting. Genetics 206, 429–438 (2017).
Van Belleghem, S. M. et al. Complex modular architecture around a simple toolkit of wing pattern genes. Nat. Ecol. Evol. 1, 52 (2017).
Gronau, I., Hubisz, M. J., Gulko, B., Danko, C. G. & Siepel, A. Bayesian inference of ancient human demography from individual genome sequences. Nat. Genet. 43, 1031–1034 (2011).
Excoffier, L., Dupanloup, I., Huerta-Sanchez, E., Sousa, V. C. & Foll, M. Robust demographic inference from genomic and SNP data. PLoS Genet. 9, e1003905 (2013).
Fritz, J. S. et al. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods 15, 461–468 (2018).
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (grant nos. 31690090 and 31690091 to Y.Z.) and the United States Department of Agriculture, Agricultural Research Service (USDA-ARS project grant no. 3091-21000-044-00D to J.Z.Y.).
Author information
Authors and Affiliations
Contributions
Y.Z. and J.Z.Y. conceived and designed the research. J.Z.Y., R.G.P. and J.E.F. selected cotton material for the experiments. Y.Z., G.H. and J.Z.Y. managed the project. M.B. and J.H. sequenced and assembled the genomes. G.H., K.W., Y.L., J.Z.Y., R.G.P. and J.E.F. prepared the samples and contributed to data analysis. G.H. and Z.W. performed the bioinformatics work. Y.Z., G.H. and Z.W. prepared the figures and tables. Y.Z., G.H., J.Z.Y., R.G.P. and J.E.F. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 High correlation of chromosome-scale assembled A1, A2 and (AD)1 genomes with Hi-C data.
a, Hi-C contact data from A1 mapped on the assembled A1-genome. b, Hi-C contact data from A2 mapped on the improved A2-genome. c, Hi-C contact data from (AD)1 mapped on the improved (AD)1-genome. The heat map represents the normalised contact matrix. The strongest and weakest contacts are shown in red and grey, respectively.
Extended Data Fig. 2 Gene synteny among our assembled A1, A2, (AD)1 genomes and previously released D5-genome16 sequences.
a, Dot plot showing gene synteny between A1 and A2 genomes. b, Dot plot showing gene synteny between A1 and D5 genomes. c, Dot plot showing gene synteny between A1 and the two subgenomes of (AD)1. d, Dot plot showing gene synteny between A2 and the two subgenomes of (AD)1. e, Dot plot showing gene synteny between A2 and D5 genomes. f, Dot plot showing gene synteny between D5 and two subgenomes of (AD)1.
Extended Data Fig. 3 Comparisons of the updated At1-subgenome with a previously reported genetic map.
a–m, Genetic versus physical map distance of the 13 chromosomes of the At1-subgenome in (AD)1. A01−A13 (a to m, respectively), the chromosomes of the At1-subgenome. The x and y axes represent the physical sequences (in megabases) and genetic distances (in centimorgans), respectively.
Extended Data Fig. 4 Comparisons of the updated Dt1-subgenome with a previously reported genetic map.
a-m, Genetic versus physical map distance of the 13 chromosomes of the Dt1-subgenome in (AD)1. D01−D13 (a to m, respectively), the chromosomes of the Dt1-subgenome. The x and y axes represent the physical sequences (in megabases) and genetic distances (in centimorgans), respectively.
Extended Data Fig. 5 Hi-C data and PCR amplification validate the border of two large inversions in chromosomes 10 and 12 between A1 and A2 genomes.
a, b, Identification of the ~42.9-Mb large inversion in chromosome 10 (a) and ~61.6-Mb large inversion in chromosome 12 (b) by Hi-C data. The upper heatmap shows a chromatin interaction matrix that maps Hi-C data from A2 against the A2-genome (A2 map to A2), and maps Hi-C data from A1 against the A2-genome (A1 map to A2). The middle panel shows a diagram of the inversion region with the four red dots representing the inversion borders. The lower heatmap shows a chromatin interaction matrix that maps Hi-C data from A1 against the A1-genome (A1 map to A1) and maps Hi-C data from A2 against the A1-genome (A2 map to A1). c, d, Validation of inversion borders in chromosomes 10 (c) and 12 (d) by PCR amplification. The forward and reverse primer sequences are shown in Supplementary Table 16. The unprocessed gel for the cropped images are presented in source data.
Extended Data Fig. 6 Phylogenetic relationship among A1, A2 and A-subgenome.
a, Distribution of mean recombination rates in protein-coding genes with available recombination rates (n = 240 genes). b, Proportion of genes with 0-20% and 80-100% of recombination rates supporting the three trees. c, Summary of phylogenetic analysis with the AU test in 10 kb windows among A1, A2, At2, D5.
Extended Data Fig. 7 A phylogenetic tree based on SNPs in the genomes with D5-genome as the outgroup.
Units (as measured by the indicated scale) show the percentage of represented polymorphic sites that differed between two individuals. Detail information of cotton accessions were described in Supplementary Table 4.
Extended Data Fig. 8 Topology weighting for A1 and A2 populations.
a, Three possible taxon topologies for A1, A2 from China group (CHG) and A2 mostly from India and Pakistan group (IPG) with D5 as the outgroup. b-n, Weightings for all three topologies plotted across the 13 chromosomes. Colour for each topology corresponds to coloring in a.
Extended Data Fig. 9 The burst of LTR/Gypsy amplification fits a Gaussian distribution and allows time estimation of burst events.
a-e, GPDF modeling data fit well with the actual TE bursts found in A1 with R2 from 0.86–0.99 (a), A2 with R2 from 0.86–0.98 (b), D5 with R2=0.94 (c), At1 with R2 from 0.95–0.99 (d) and Dt1 with R2 from 0.91–0.93 (e). The estimated time of the LTR/Gypsy burst event associated with each genome or subgenome is shown in the graphs. The peak width was defined as 2.58σ that covers >99.5% of the nucleotide substitution events in TEs.
Extended Data Fig. 10 Volcano plots for A1-At1 gene expression in elongating fibers at 15 DPA.
Each hollow point represents a gene and genes with SVs within 5 kb of their start or stop codons are indicated by a triangle. The dashed lines show the thresholds (P-value ≤ 0.001 and two-fold change).
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Tables 1–5, 15 and 16
Supplementary Table
Supplementary Tables 6–14
Source data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 5
Unprocessed gels for Extended Data Fig. 5
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, G., Wu, Z., Percy, R.G. et al. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat Genet 52, 516–524 (2020). https://doi.org/10.1038/s41588-020-0607-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-020-0607-4
This article is cited by
-
An insight into the gene expression evolution in Gossypium species based on the leaf transcriptomes
BMC Genomics (2024)
-
Genome-wide identification and expression profiling of photosystem II (PsbX) gene family in upland cotton (Gossypium hirsutum L)
Journal of Cotton Research (2024)
-
Molecular characterization and expression pattern of Rubisco activase gene GhRCAβ2 in upland cotton (Gossypium hirsutum L.)
Genes & Genomics (2024)
-
Targeted genome editing for cotton improvement: prospects and challenges
The Nucleus (2024)
-
The GaKAN2, a KANADI transcription factor, modulates stem trichomes in Gossypium arboreum
Molecular Genetics and Genomics (2024)