Abstract
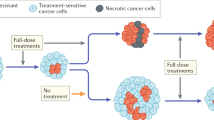
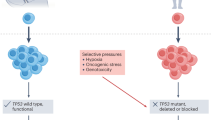
Local adaptation directs populations towards environment-specific fitness maxima through acquisition of positively selected traits. However, rapid environmental changes can identify hidden fitness trade-offs that turn adaptation into maladaptation, resulting in evolutionary traps. Cancer, a disease that is prone to drug resistance, is in principle susceptible to such traps. We therefore performed pooled CRISPR–Cas9 knockout screens in acute myeloid leukemia (AML) cells treated with various chemotherapies to map the drug-dependent genetic basis of fitness trade-offs, a concept known as antagonistic pleiotropy (AP). We identified a PRC2–NSD2/3-mediated MYC regulatory axis as a drug-induced AP pathway whose ability to confer resistance to bromodomain inhibition and sensitivity to BCL-2 inhibition templates an evolutionary trap. Across diverse AML cell-line and patient-derived xenograft models, we find that acquisition of resistance to bromodomain inhibition through this pathway exposes coincident hypersensitivity to BCL-2 inhibition. Thus, drug-induced AP can be leveraged to design evolutionary traps that selectively target drug resistance in cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
Scripts for analyzing CRISPR–Cas9 screens and calculating API are available on Github (https://github.com/linkvein/).
References
Kawecki, T. J. & Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241 (2004).
Singer, M. C. & Parmesan, C. Lethal trap created by adaptive evolutionary response to an exotic resource. Nature 557, 238–241 (2018).
Schlaepfer, M. A., Runge, M. C. & Sherman, P. W. Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480 (2002).
Robertson, B. A., Rehage, J. S. & Sih, A. Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 28, 552–560 (2013).
Walther, V. et al. Can oncology recapitulate paleontology? Lessons from species extinctions. Nat. Rev. Clin. Oncol. 12, 273–285 (2015).
Van Allen, E. M. et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 4, 94–109 (2014).
Singleton, K. R. et al. Melanoma therapeutic strategies that select against resistance by exploiting MYC-driven evolutionary convergence. Cell Rep. 21, 2796–2812 (2017).
Holohan, C. et al. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Gatenby, R. & Brown, J. The evolution and ecology of resistance in cancer therapy. Cold Spring Harb. Perspect. Med. 8, 3 (2018).
Konieczkowski, D. J., Johannessen, C. M. & Garraway, L. A. A convergence-based framework for cancer drug resistance. Cancer Cell 33, 801–815 (2018).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Juric, D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Wang, L. et al. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell 173, 1413–1425.e14 (2018).
Chen, G. et al. Targeting the adaptability of heterogeneous aneuploids. Cell 160, 771–784 (2015).
Imamovic, L. et al. Drug-driven phenotypic convergence supports rational treatment strategies of chronic infections. Cell 172, 121–134.e14 (2018).
Amirouchene-Angelozzi, N., Swanton, C. & Bardelli, A. Tumor evolution as a therapeutic target. Cancer Discov. 7, 805–817 (2017).
Zhao, B. et al. Exploiting temporal collateral sensitivity in tumor clonal evolution. Cell 165, 234–246 (2016).
Savolainen, O., Lascoux, M. & Merila, J. Ecological genomics of local adaptation. Nat. Rev. Genet. 14, 807–820 (2013).
Tiffin, P. & Ross-Ibarra, J. Advances and limits of using population genetics to understand local adaptation. Trends Ecol. Evol. 29, 673–680 (2014).
Hart, T. et al. Measuring error rates in genomic perturbation screens: gold standards for human functional genomics. Mol. Syst. Biol. 10, 733 (2014).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Lin, K. H. et al. Systematic Dissection of the Metabolic-Apoptotic Interface in AML Reveals Heme Biosynthesis to Be a Regulator of Drug Sensitivity. Cell Metab. 29, 1217–1231.e7 (2019).
Fiskus, W. et al. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia 28, 2155–2164 (2014).
Fiskus, W. et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood 114, 2733–2743 (2009).
Beaumont, K. A. et al. Cell cycle phase-specific drug resistance as an escape mechanism of melanoma cells. J. Invest. Dermatol. 136, 1479–1489 (2016).
Knutson, S. K. et al. Synergistic anti-tumor activity of EZH2 inhibitors and glucocorticoid receptor agonists in models of germinal center non-Hodgkin lymphomas. PLoS ONE 9, e111840 (2014).
Lee, T., Karon, M. & Momparler, R. L. Kinetic studies on phosphorylation of 5-azacytidine with the purified uridine-cytidine kinase from calf thymus. Cancer Res. 34, 2482–2488 (1974).
Liliemark, J. O., Plunkett, W. & Dixon, D. O. Relationship of 1-β-d-arabinofuranosylcytosine in plasma to 1-β-d-arabinofuranosylcytosine 5′-triphosphate levels in leukemic cells during treatment with high-dose 1-β-d-arabinofuranosylcytosine. Cancer Res. 45, 5952–5957 (1985).
Cai, J. et al. Two distinct molecular mechanisms underlying cytarabine resistance in human leukemic cells. Cancer Res. 68, 2349–2357 (2008).
Margueron, R. & Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349 (2011).
Holoch, D. & Margueron, R. Mechanisms regulating PRC2 recruitment and enzymatic activity. Trends Biochem. Sci. 42, 531–542 (2017).
Schmitges, F. W. et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Mol Cell 42, 330–341 (2011).
Zheng, Y. et al. Total kinetic analysis reveals how combinatorial methylation patterns are established on lysines 27 and 36 of histone H3. Proc. Natl Acad. Sci. USA 109, 13549–13554 (2012).
Bennett, R. L. et al. The role of nuclear receptor-binding SET domain family histone lysine methyltransferases in cancer. Cold Spring Harb. Perspect. Med. 7, a026708 (2017).
Kaur, M. & Cole, M. D. MYC acts via the PTEN tumor suppressor to elicit autoregulation and genome-wide gene repression by activation of the Ezh2 methyltransferase. Cancer Res. 73, 695–705 (2013).
Lin, K. H. et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci. Rep. 6, 27696 (2016).
Ramsey, H. E. et al. A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 8, 1566–1581 (2018).
Gollner, S. et al. Loss of the histone methyltransferase EZH2 induces resistance to multiple drugs in acute myeloid leukemia. Nat. Med. 23, 69–78 (2017).
Farrell, A. S. & Sears, R. C. MYC degradation. Cold Spring Harb. Perspect. Med. 4, a014365 (2014).
Bradley, W. D. et al. EZH2 inhibitor efficacy in non-Hodgkin’s lymphoma does not require suppression of H3K27 monomethylation. Chem. Biol. 21, 1463–1475 (2014).
Ryan, J. & Letai, A. BH3 profiling in whole cells by fluorimeter or FACS. Methods 61, 156–164 (2013).
Campone, M. et al. c-Myc dependent expression of pro-apoptotic Bim renders HER2-overexpressing breast cancer cells dependent on anti-apoptotic Mcl-1. Mol. Cancer 10, 110 (2011).
Lee, Y. Y. et al. CREB-binding protein (CBP) regulates β-adrenoceptor (β-AR)-mediated apoptosis. Cell Death Differ. 20, 941–952 (2013).
Muthalagu, N. et al. BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues. Cell Rep. 8, 1347–1353 (2014).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Ni Chonghaile, T. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011).
Dauch, D. et al. A MYC-aurora kinase A protein complex represents an actionable drug target in p53-altered liver cancer. Nat. Med. 22, 744–753 (2016).
Stine, Z. E. et al. MYC, metabolism, and cancer. Cancer Discov. 5, 1024–1039 (2015).
den Hollander, J. et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood 116, 1498–1505 (2010).
Fong, C. Y. et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature 525, 538–542 (2015).
Rathert, P. et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature 525, 543–547 (2015).
Shu, S. et al. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature 529, 413–417 (2016).
Xia, B. et al. c-Myc plays part in drug resistance mediated by bone marrow stromal cells in acute myeloid leukemia. Leuk. Res. 39, 92–99 (2015).
Zhang, Y. et al. Sp1 and c-Myc modulate drug resistance of leukemia stem cells by regulating survivin expression through the ERK-MSK MAPK signaling pathway. Mol. Cancer 14, 56 (2015).
Pan, X. N. et al. Inhibition of c-Myc overcomes cytotoxic drug resistance in acute myeloid leukemia cells by promoting differentiation. PLoS ONE 9, e105381 (2014).
Cortes, J. E. et al. Glasdegib plus intensive/nonintensive chemotherapy in untreated acute myeloid leukemia: BRIGHT AML 1019 Phase III trials. Future Oncol. 15, 3531–3545 (2019).
Cortes, J. E. et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 33, 379–389 (2019).
DiNardo, C. D. et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133, 7–17 (2019).
Kotschy, A. et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 538, 477–482 (2016).
Leverson, J. D. et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 7, 279ra40 (2015).
Soderquist, R. S. et al. Systematic mapping of BCL-2 gene dependencies in cancer reveals molecular determinants of BH3 mimetic sensitivity. Nat. Commun. 9, 3513 (2018).
Xu, Y. & Vakoc, C. R. Targeting cancer cells with BET bromodomain inhibitors. Cold Spring Harb. Perspect. Med. 7, a026674 (2017).
Stathis, A. & Bertoni, F. BET proteins as targets for anticancer treatment. Cancer Discov. 8, 24–36 (2018).
Berthon, C. et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 3, e186–e195 (2016).
Fiskus, W. et al. Superior efficacy of cotreatment with BET protein inhibitor and BCL2 or MCL1 inhibitor against AML blast progenitor cells. Blood Cancer J. 9, 4 (2019).
Esteve-Arenys, A. et al. The BET bromodomain inhibitor CPI203 overcomes resistance to ABT-199 (venetoclax) by downregulation of BFL-1/A1 in in vitro and in vivo models of MYC+/BCL2+ double hit lymphoma. Oncogene 37, 1830–1844 (2018).
Wang, T. et al. Genetic screens in human cells using the CRISPR–Cas9 system. Science 343, 80–84 (2014).
Sarosiek, K. A. et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell 51, 751–765 (2013).
Vo, T. T. et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012).
Acknowledgements
We thank the members of the K.C.W. laboratory and A. Puissant laboratory for helpful discussions and scientific input. We also thank K. Wood (University of Michigan), G. Blobe and S. Floyd (Duke Pharmacology & Cancer Biology) for providing helpful feedback. This work was supported by Duke University School of Medicine start-up funds and support from the Duke Cancer Institute (K.C.W.), NIH awards (R01CA207083 to K.C.W., F30CA206348 to K.H.L. and F31CA195967 to P.S.W.), National Science Foundation Graduate Research Fellowship awards (DGE-1106401 to G.R.A. and DGF-1106401 to L.C.), the Duke Medical Scientist Training Program (T32 GM007171 to K.H.L.), the Duke Undergraduate Research Support Office (to J.C.R. and A.X.), the ATIP/AVENIR French research program (to A.P.) and the EHA research grant for Non-Clinical Advanced Fellow (to A.P.). A.P. is a recipient of support from the ERC Starting program (758848) and supported by the St Louis Association for leukemia research. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation or the NIH. Finally, we dedicate this work to the memory of our friend, Kimberly Brigati Wang, and her courageous fight against AML.
Author information
Authors and Affiliations
Contributions
K.H.L., J.C.R., A.P. and K.C.W. conceptualized the project. K.H.L. and J.C.R. were responsible for methodology. Validation was done by K.H.L. and J.C.R. K.H.L., J.C.R., A.X., Z.D. and E.T.W. performed the formal analysis. The investigation was carried out by K.H.L., J.C.R., A.X., Y.-R.A., B.P., R.D.B., A.F. and R.I. Resources were collected by Y.-R.A., R.T.S., G.R.A., K.R.S., A.E.D., P.S.W., A.P. and K.C.W. Data were curated by K.H.L., J.C.R., A.X. and J.W.L. The original draft was written by K.H.L., J.C.R. and K.C.W. All authors reviewed and edited the paper. K.H.L. and J.C.R. were responsible for visualization. L.C., A.P. and K.C.W. supervised the project. Funding was acquired by K.H.L., G.R.A., P.S.W., A.P. and K.C.W.
Corresponding authors
Ethics declarations
Competing interests
J.W.L. serves on the scientific advisory board and owns equity in Nanocare Technologies and Raphael Pharmaceuticals. R.I. has received previous funding from Oncoethix SA for work on the bromodomain inhibitor OTX015. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Validation of API using external datasets.
a, API analysis performed on published CRISPR/Cas9-based gene essentiality dataset identifies overrepresented gene ontologies in top 15% of AP genes across 14 AML cell lines. b–f, Exemplar gene networks associated with overrepresented gene ontologies in (a). g, API analysis performed on published shRNA-based gene essentiality dataset identifies overrepresented gene ontologies in top 15% of AP genes across 398 human cancer cell lines. h–j, Exemplar gene networks associated with overrepresented gene ontologies in (g). k, Cell line lineages represented in published shRNA-based gene essentiality dataset plotted according to number of AP genes versus number of cell lines within each lineage. Red dashed line depicts number of AP genes with random sampling of cell lines for a given n. l, Cell line lineages plotted according to fraction of expected AP, defined as the number of AP genes in a given linage divided by number of expected AP genes for a given n. b–f; h–j, Heatmaps generated by unsupervised hierarchical clustering of genes (columns) and cell lines (rows) based on Euclidean distance.
Extended Data Fig. 2 Additional analysis of drug-treated screens using API.
a, Graphical depiction of scoring regions of nine drug modifier screens. Red lines indicate cutoff controlled at p-value 0.05. b, Gene ontology analysis of drug-induced AP genes ranked by fold-change. c, Circos plot displaying data from drug-modifier CRISPR screens as in Fig. 2a. d, PCA analysis of nine drug modifier screens conducted in n = 2 biologically independent experiments. Colors denote different drugs. e, Heatmap representing effect of sgRNAs targeting DCK, UCK2, SLC29A1 on cytarabine, decitabine, and azacitidine; schematic depicts effect of DCK and SLC29A1 on deoxycytidines. f, Correlogram depicting Pearson correlation coefficients of DCK, SLC29A1, and UCK2 depletion across nine drug modifier screens. g,h, Decitabine (g) and cytarabine (h) 8-point drug dilution series following CRISPR/Cas9 knockout of SLC29A1 or DCK versus non-targeting control in OCI-AML2 cells. i,j, Fold-change of SLC29A1 (i) and DCK (j) transcripts following CRISPR/Cas9 knockout of SLC29A1 versus non-targeting control. P-values computed by two-sided two-sample t-Test for equal means. g–j, Data are mean ± SEM for n = 3 biologically independent experiments.
Extended Data Fig. 3 Comparisons between API, PCA, and correlation.
a, List of 36 drug pairs ranked by greatest PCA distance (left), greatest % of shared AP interactions (middle), and smallest Pearson correlation coefficient (right). Lines match drug pairs in each list. Drug pairs >10 positions lower in percent of shared AP interactions rank joined by red lines; drug pairs >10 positions higher in percent of shared AP interactions rank joined by blue lines. b, Percent of shared AP interactions, Pearson correlation coefficient and PCA distance for 36 drug pairs. a,b, Data from drug-modifier screens conducted in n = 2 biologically independent experiments.
Extended Data Fig. 4 KDM1A functions as a drug-induced AP gene by regulating differentiation.
a, Correlogram depicting Pearson correlation coefficients of KDM1A and RCOR1/2/3 depletion across nine drug modifier screens. Data from drug-modifier screens conducted in n = 2 biologically independent experiments. b, Immunoblot analysis of LSD1, MYC, H3K4me1 and H3K9me2 following CRISPR/Cas9 knockout of KDM1A in OCI-AML2 cells. Representative immunoblot of n = 3 independent experiments. Uncropped blots in Source Data. c, BH3 profiling of OCI-AML2 cells following CRISPR/Cas9 knockout of KDM1A versus non-targeting control. BCL2 priming defined as percent depolarization from HRK peptide (10μM) subtracted from percent depolarization from BAD (10μM) peptide. d,e, Flow-cytometry analysis of CD11b expression distribution (d) and median signal (e) in OCI-AML2 cells following CRISPR/Cas9 knockout of KDM1A versus non-targeting control. Median signal normalized to non-targeting control sgRNA. Data are mean ± SEM for n = 2 biologically independent experiments. f–i, BCL2 (f, h) and CD11b (encoded by ITGAM) (g, i) expression in hematopoietic stem cells (HSC; n = 11), common myeloid progenitors (CMP, n = 3), megakaryocyte-erythroid progenitor cell (MEP, n = 3), granulocyte monocyte progenitors (GMP, n = 3), CD11c+ myeloid dendritic cells (mDC, n = 5), CD123+ plasmacytoid dendritic cells (pDC, n = 5), and CD14+ monocytes (n = 13) from BloodSpot using HemaExplorer dataset. Sample size refers to biologically independent samples. Data are log2 expression of highest intensity microarray probe. Boxplot elements defined in Methods. c,f,g; P-values computed by two-sided two-sample t-Test for equal means. c,e,f,g; Data are mean ± SEM for n = 3 biologically independent experiments.
Extended Data Fig. 5 MYC is a drug-induced AP gene.
a, Correlogram depicting Pearson correlation coefficients of MYC, NSD3, NSD2, EED and EZH2 depletion values across nine drug modifier screens. Data from drug-modifier screens conducted in n = 2 biologically independent experiments. b,c, Confirmation of MYC shRNA knockdown in OCI-AML2 by transcript (b) and protein (c). Representative immunoblot of n = 3 independent experiments. d,e, JQ-1 (d) and ABT-199 (e) 8-point drug dilution series following shRNA knockdown of MYC in OCI-AML2 cells. f,g, Confirmation of MYC overexpression in OCI-AML2 by transcript (f) and protein (g). Representative immunoblot of n = 3 independent experiments. h,i, JQ-1 (h) and ABT-199 (i) 8-point drug dilution series following overexpression of MYC in OCI-AML2 cells. b,f; P-values computed by two-sided two-sample t-Test for equal means. b,d–f,h,i, Data are mean ± SEM for n = 3 biologically independent experiments. Uncropped blots in Source Data.
Extended Data Fig. 6 EZH2/EED/NSD2/NSD3 modulate JQ-1 and ABT-199 sensitivity through MYC.
a,b; d,e, Relative expression of NSD2 (a), NSD3 (b), EZH2 (d), and EED (e) transcripts in cells with CRISPR/Cas9 knockout. P-values computed by two-sided two-sample t-Test for equal means. Data are presented as mean ± SEM for n = 3 biologically independent experiments. c,f, 8-point dose-response curves of JQ-1 (c) and ABT-199 (f) following CRISPR/Cas9 knockout of NSD2/3 (c) and EZH2 or EED (f). Data are presented as mean ± SEM for n = 3 biologically independent experiments.
Extended Data Fig. 7 Additional characterization of ABT-199 and JQ-1-resistant AML cells.
a, 8-point dose-response curves of ABT-199 in parental and ABT-199-resistant OCI-AML2 cells. b, Immunoblot analysis of EZH2 and MYC in parental and ABT-199-resistant OCI-AML2 cells. c, Fold-change of MYC transcripts across matched parental and JQ-1-resistant AML cell lines. d, Immunoblot analysis of NSD2 and NSD3 across matched parental and JQ-1 resistant AML cell lines. e,f, ARV771 GI50 values of parental and JQ-1-resistant OCI-AML2 (e) and MOLM-13 (f). g, Immunoblot analysis of phosphorylated EZH2 at T487 and S21 in parental and JQ-1 resistant OCI-AML2 cells. h, Immunoblot analysis of ubiquitin and EZH2 following immunoprecipitation of EZH2 in OCI-AML2 cells. i, Immunoblot analysis of EZH2 following treatment of JQ-1 resistant OCI-AML2 cells with CDK1 inhibitor (CDK1i) or proteasome inhibitor (Bortezomib) for 24 hours. a,c,e,f; Data are mean ± SEM for n = 3 biologically independent experiments. c,e,f; P-values computed by two-sided two-sample t-Test for equal means. b,d,g–i; Representative immunoblots of n = 3 independent experiments. Uncropped blots in Source Data.
Extended Data Fig. 8 MYC upregulation in JQ-1-resistant cells can be driven by AKT/ERK.
a,b, Immunoblot analysis of MYC (a) in matched parental and JQ-1 resistant AML cells following treatment with 20μg/mL cycloheximide (CHX) for indicated times. Quantification by densitometry (b) normalized to time zero signal. c, Immunoblot analysis of phosphorylated ERK at T202/204 and phosphorylated AKT at S437 in parental and JQ-1 resistant AML cells relative to total proteins. d, Immunoblot analysis of MYC in parental and JQ-1 resistant OCI-AML2 and MV4;11 cells treated with VX11E for 24 hours. OCI-AML2 cells treated with 500nM VX11E and MV4;11 cells treated with 2μM VX11E. e,f, GI50 value of VX11E in combination with 100nM JQ-1 normalized to VX11E alone in parental and JQ-1 resistant OCI-AML2 (e) and MV4;11 (f) cells. g,h, Immunoblot analysis of MYC, NSD2 (g) and NSD3 (h) in OCI-AML2 cells following overexpression of pCDH-MYC in combination with sgRNAs targeting NSD2 (g) and NSD3 (h). i, ABT-199 8-point drug dilution series following shRNA knockdown of MYC in combination with GSK-126. e,f,i; Data are mean ± SEM for n = 3 biologically independent experiments. e,f; P-values computed by two-sided two-sample t-Test for equal means. a,c,d,g,h; Representative immunoblots of n = 3 independent experiments. Uncropped blots in Source Data.
Extended Data Fig. 9 JQ-1-resistant AML cells harbor widespread BIM-related collateral sensitivities.
a, 8-point dose-response curves of JQ-1 in parental and ABT-199-resistant OCI-AML2 cells. b, 8-point dose-response curves of ABT-199 in JQ-1-resistant OCI-AML2 cells following shRNA knockdown of MYC. c,d, Effect of 72-hour, 200nM JQ-1 treatment on cell viability of parental and JQ-1 resistant OCI-AML2 cultured continuously in JQ-1 (c) or taken off JQ-1 for 10 days (d), normalized to effect of vehicle treatment. e,f, Effect of 72-hour, 2nM ABT-199 treatment on cell viability of parental and JQ-1-resistant OCI-AML2 cells cultured continuously in JQ-1 (e) or taken off JQ-1 for 10 days (f), normalized to effect of vehicle treatment. g, Fold-change of BIM transcripts across matched parental and JQ-1 resistant AML cell lines. h, Immunoblot of MYC and BIM following overexpression of pCDH-MYC in OCI-AML2; representative of n = 1 independent experiments. Uncropped blots in Source Data. i, ABT-199 GI50 in parental and JQ-1 resistant MOLM-13 cells following CRISPR/Cas9 knockout of BIM or non-targeting control. j, Specification of 40 compound drug screen in JQ-1 resistant OCI-AML2 cells relative to parental. k–p, 8-point dose-response curves in parental and drug resistant cell line derivatives. q, Gating strategy for flow cytometric analysis of murine bone marrow aspirate. c–g; i, P-values computed by two-sided two-sample t-Test for equal means. a–g; i–p, Data are mean ± SEM for n = 3 biologically independent experiments.
Supplementary information
Supplementary Information
Supplementary Note
Supplementary Tables
Supplementary Tables 1–5
Source data
Source Data Fig. 2
Statistical source data
Source Data Fig. 3
Unprocessed western blots
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Unprocessed western blots
Source Data Fig. 4
Statistical source data
Source Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 2
Statistical source data
Source Data Extended Data Fig. 4
Unprocessed western blots
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 5
Unprocessed western blots
Source Data Extended Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 6
Statistical source data
Source Data Extended Data Fig. 7
Unprocessed western blots
Source Data Extended Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 8
Unprocessed western blots
Source Data Extended Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 9
Unprocessed western blots
Source Data Extended Data Fig. 9
Statistical source data
Rights and permissions
About this article
Cite this article
Lin, K.H., Rutter, J.C., Xie, A. et al. Using antagonistic pleiotropy to design a chemotherapy-induced evolutionary trap to target drug resistance in cancer. Nat Genet 52, 408–417 (2020). https://doi.org/10.1038/s41588-020-0590-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-020-0590-9
This article is cited by
-
Drug dependence in cancer is exploitable by optimally constructed treatment holidays
Nature Ecology & Evolution (2023)
-
Molecular evolutionary process of advanced gastric cancer during sequential chemotherapy detected by circulating tumor DNA
Journal of Translational Medicine (2022)
-
Clinical utility of PDX cohorts to reveal biomarkers of intrinsic resistance and clonal architecture changes underlying acquired resistance to cetuximab in HNSCC
Signal Transduction and Targeted Therapy (2022)
-
The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs
Nature Reviews Cancer (2022)
-
P2RY2-AKT activation is a therapeutically actionable consequence of XPO1 inhibition in acute myeloid leukemia
Nature Cancer (2022)