Abstract
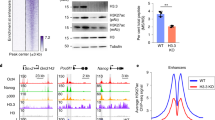
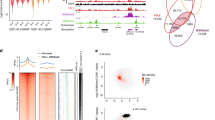
Mutations in enzymes that modify histone H3 at lysine 4 (H3K4) or lysine 36 (H3K36) have been linked to human disease, yet the role of these residues in mammals is unclear. We mutated K4 or K36 to alanine in the histone variant H3.3 and showed that the K4A mutation in mouse embryonic stem cells (ESCs) impaired differentiation and induced widespread gene expression changes. K4A resulted in substantial H3.3 depletion, especially at ESC promoters; it was accompanied by reduced remodeler binding and increased RNA polymerase II (Pol II) activity. Regulatory regions depleted of H3.3K4A showed histone modification alterations and changes in enhancer activity that correlated with gene expression. In contrast, the K36A mutation did not alter H3.3 deposition and affected gene expression at the later stages of differentiation. Thus, H3K4 is required for nucleosome deposition, histone turnover and chromatin remodeler binding at regulatory regions, where tight regulation of Pol II activity is necessary for proper ESC differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The next-generation sequencing datasets (RNA-seq, ChIP–seq and PRO-seq) have been deposited in the ArrayExpress database under accession numbers E-MTAB-6821 (RNA-seq), E-MTAB-6822 and E-MTAB-8614 (ChIP–seq), E-MTAB-8617 (PRO-seq). The processed mass spectrometry data for the peptide pulldown experiment can be found in Supplementary Table 1. The mass spectrometry data used to measure histone modifications in ESCs can be found in Supplementary Table 3. The mass spectrometry raw files have been uploaded to https://chorusproject.org/ under project number 1637. Additional data supporting the findings of this study are available from the corresponding author upon request. Source data (full scans of the immunoblots) for Figs. 3 and 4 and Extended Data Figs. 6–8 are provided with the paper.
Code availability
We have made use of publicly available software and tools. All code used to analyze the data in this study is available from the corresponding author upon request.
References
Li, B., Carey, M. & Workman, J. L. The role of chromatin during transcription. Cell 128, 707–719 (2007).
Elsaesser, S. J., Goldberg, A. D. & Allis, C. D. New functions for an old variant: no substitute for histone H3.3. Curr. Opin. Genet. Dev. 20, 110–117 (2010).
Ahmad, K. & Henikoff, S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200 (2002).
Kuo, A. J. et al. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol. Cell 44, 609–620 (2011).
Nimura, K. et al. A histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf–Hirschhorn syndrome. Nature 460, 287–291 (2009).
Shilatifard, A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 81, 65–95 (2012).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Jang, C.-W., Shibata, Y., Starmer, J., Yee, D. & Magnuson, T. Histone H3.3 maintains genome integrity during mammalian development. Genes Dev. 29, 1377–1392 (2015).
Tang, M. C. W. et al. Contribution of the two genes encoding histone variant h3.3 to viability and fertility in mice. PLoS Genet. 11, e1004964 (2015).
Maze, I. et al. Critical role of histone turnover in neuronal transcription and plasticity. Neuron 87, 77–94 (2015).
Banaszynski, L. A. et al. Hira-dependent histone H3.3 deposition facilitates PRC2 recruitment at developmental loci in ES cells. Cell 155, 107–120 (2013).
Herz, H.-M. et al. Histone H3 lysine-to-methionine mutants as a paradigm to study chromatin signaling. Science 345, 1065–1070 (2014).
Chan, K.-M. et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 27, 985–990 (2013).
Lu, C. et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science 352, 844–849 (2016).
Streubel, G. et al. The H3K36me2 methyltransferase Nsd1 demarcates PRC2-mediated H3K27me2 and H3K27me3 domains in embryonic stem cells. Mol. Cell 70, 371–379.e5 (2018).
Luco, R. F. et al. Regulation of alternative splicing by histone modifications. Science 327, 996–1000 (2010).
Wagner, E. J. & Carpenter, P. B. Understanding the language of Lys36 methylation at histone H3. Nat. Rev. Mol. Cell Biol. 13, 115–126 (2012).
Meers, M. P. et al. Histone gene replacement reveals a post-transcriptional role for H3K36 in maintaining metazoan transcriptome fidelity. eLife 6, e23249 (2017).
Shen, Y. et al. A map of the cis-regulatory sequences in the mouse genome. Nature 488, 116–120 (2012).
Deaton, A. M. et al. Enhancer regions show high histone H3.3 turnover that changes during differentiation. eLife 5, e15316 (2016).
Ricketts, M. D. et al. Ubinuclein-1 confers histone H3.3-specific-binding by the HIRA histone chaperone complex. Nat. Commun. 6, 7711 (2015).
Goldberg, A. D. et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691 (2010).
Lewis, P. W., Elsaesser, S. J., Noh, K.-M., Stadler, S. C. & Allis, C. D. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl Acad. Sci. USA 107, 14075–14080 (2010).
de Dieuleveult, M. et al. Genome-wide nucleosome specificity and function of chromatin remodellers in ES cells. Nature 530, 113–116 (2016).
Bornelöv, S. et al. The nucleosome remodeling and deacetylation complex modulates chromatin structure at sites of active transcription to fine-tune gene expression. Mol. Cell 71, 56–72.e4 (2018).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Zhao, H. et al. The chromatin remodeler Chd4 maintains embryonic stem cell identity by controlling pluripotency- and differentiation-associated genes. J. Biol. Chem. 292, 8507–8519 (2017).
Martire, S. et al. Phosphorylation of histone H3.3 at serine 31 promotes p300 activity and enhancer acetylation. Nat. Genet. 51, 941–946 (2019).
Clapier, C. R., Iwasa, J., Cairns, B. R. & Peterson, C. L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 18, 407–422 (2017).
Kraushaar, D. C. et al. The gene repressor complex NuRD interacts with the histone variant H3.3 at promoters of active genes. Genome Res. 28, 1646–1655 (2018).
Hödl, M. & Basler, K. Transcription in the absence of histone H3.2 and H3K4 methylation. Curr. Biol. 22, 2253–2257 (2012).
Dai, J. et al. Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell 134, 1066–1078 (2008).
Fang, D. et al. The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas. Science 352, 1344–1348 (2016).
Lewis, P. W. et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340, 857–861 (2013).
Nacev, B. A. et al. The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature 567, 473–478 (2019).
Behjati, S. et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 45, 1479–1482 (2013).
Sidoli, S. et al. Middle-down hybrid chromatography/tandem mass spectrometry workflow for characterization of combinatorial post-translational modifications in histones. Proteomics 14, 2200–2211 (2014).
Bibel, M., Richter, J., Lacroix, E. & Barde, Y.-A. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat. Protoc. 2, 1034–1043 (2007).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Chu, V. T. Increasing the efficiency of homology-directed repair for CRISPR–Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548 (2015).
Maruyama, T. et al. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33, 538–542 (2015).
Gehre, M. et al. Efficient strategies to detect genome editing and integrity in CRISPR-Cas9 engineered ESCs. Preprint at bioRxiv https://doi.org/10.1101/635151 (2019).
Anders, S., Reyes, A. & Huber, W. Detecting differential usage of exons from RNA-seq data. Genome Res. 22, 2008–2017 (2012).
Iacovino, M. et al. Inducible cassette exchange: a rapid and efficient system enabling conditional gene expression in embryonic stem and primary cells. Stem Cells 29, 1580–1588 (2011).
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Xu, S., Grullon, S., Ge, K. & Peng, W. Spatial clustering for identification of ChIP-enriched regions (SICER) to map regions of histone methylation patterns in embryonic stem cells. Methods Mol. Biol. 1150, 97–111 (2014).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Shen, L., Shao, N., Liu, X. & Nestler, E. ngs.plot: quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics 15, 284 (2014).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Mahat, D. B. et al. Base-pair-resolution genome-wide mapping of active RNA polymerases using precision nuclear run-on (PRO-seq). Nat. Protoc. 11, 1455–1476 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Wang, Y. et al. ISL1 and JMJD3 synergistically control cardiac differentiation of embryonic stem cells. Nucleic Acids Res. 44, 6741–6755 (2016).
Takahashi, T. et al. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 107, 1912–1916 (2003).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Sidoli, S. & Garcia, B. A. Characterization of individual histone posttranslational modifications and their combinatorial patterns by mass spectrometry-based proteomics strategies. Methods Mol. Biol. 1528, 121–148 (2017).
Sidoli, S. et al. Metabolic labeling in middle-down proteomics allows for investigation of the dynamics of the histone code. Epigenetics Chromatin 10, 34 (2017).
Perez-Pinera, P., Ousterout, D. G., Brown, M. T. & Gersbach, C. A. Gene targeting to the ROSA26 locus directed by engineered zinc finger nucleases. Nucleic Acids Res. 40, 3741–3752 (2012).
Chu, V. T. et al. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol. 16, 4 (2016).
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016).
Noh, K.-M. et al. Engineering of a histone-recognition domain in Dnmt3a alters the epigenetic landscape and phenotypic features of mouse ESCs. Mol. Cell 59, 89–103 (2015).
Conrad, T., Marsico, A., Gehre, M. & Orom, U. A. Microprocessor activity controls differential miRNA biogenesis in vivo. Cell Rep. 9, 542–554 (2014).
Acknowledgements
We thank the staff at the EMBL Genomics Core Facility, the Flow Cytometry Core Facility, the Proteomics Core Facility and the Advanced Light Microscopy Facility for sample preparation and data generation. We thank C. Girardot and the Genome Biology Computational Support for their assistance in data analysis, N. Arecco for establishing the PRO-seq experiment, A. Hill for help with image analysis and E. Furlong and the Noh laboratory for revising the manuscript and for helpful discussions. This work is supported by the DFG fund (no. SPP 1738-NO 1249 to K.M.N.) and the EMBL Interdisciplinary Postdoc (EI3POD) fellowship under Marie Skłodowska-Curie Actions COFUND (no. 664726 to D.B.). S.S. and B.A.G. gratefully acknowledge National Institutes of Helth grant nos. CA196539, GM110174 and AI118891.
Author information
Authors and Affiliations
Contributions
M.G. and K.-M.N. conceived the project. M.G. and K.-M.N. designed the experiments. M.G., S.S., M.J.L., M.T. and N.D. collected and analyzed the data. M.G., D.B. and J.B.Z. performed the bioinformatics analyses. B.A.G. provided essential resources and reagents. M.G. and K.-M.N. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Validation of CRISPR gene editing of H3.3 in ESCs.
a, General scheme used to introduce point-mutations into H3.3B. Guide sequences were designed to target Cas9 to the first coding exon of H3.3B close to the mutation site. The single-stranded DNA repair template (ssODN) contains nucleotide changes to introduce lysine-to-alanine mutation and 3 additional synonymous mutations inside the sequence complementary to the guide or inside the PAM to prevent re-cleavage after repair. Optionally, synonymous mutations can give rise to a new restriction site used to validate clones. b, Example of a restriction digest using a newly introduced restriction site after CRISPR editing. Genomic DNA of wildtype cells and a mutant cell line was used for PCR amplification followed by restriction digest with BanI. Experiments were repeated at least three times independently with similar results. c, Sanger-sequencing results of the H3.3B locus for H3.3K4A and H3.3K36A mutant cells. Blat tool is used to compare the results with the wildtype genome (mm10) and the search is visualized using UCSC. Zoom into the region of the first coding exon reveals successful introduction of lysine-to-alanine mutation at either K4 or K36, respectively, and the introduction of 3 additional synonymous mutations inside guide recognition site or PAM. d, Analysis of chromatograms from Sanger sequencing confirms the homozygous exchange of targeted nucleotides in H3.3K4A/K36A mutant cell lines. e, Normalized RNA-seq counts of H3.3A (H3f3a) expression in controls, H3.3K4A/K36A ESCs in comparison to wild type ESCs, confirming knockout of H3.3A.
Extended Data Fig. 2 Off-target analysis of generated CRISPR clones.
a, Three predicted off-target sites for CRISPR guides used to introduce K4A and K36A mutations were amplified by PCR and analyzed by Sanger-Sequencing to confirm the absence of indels. b-d, Off-target analysis of two controls in b, three H3.3K4A in c, or three H3.3K36A in d, mutant cell lines to confirm their genomic integrity after CRISPR targeting. log2(FoldChanges) of gene expression in CRISPR clones compared to unmodified wild type ESCs were determined by DESeq2 and plotted over chromosome position. Lines indicate CIRSPR cleavage site inside H3f3a gene (yellow) and H3f3b gene (blue). Loss or duplication of a chromosome part can be detected by coordinated up- or down-regulation of close-by genes. All clones included in this study do not have detectable chromosome deletion/duplications.
Extended Data Fig. 3 Cell cycle and apoptosis analysis of K4A and K36A clones.
a, Representative bright-field images of ESC colonies grown on gelatin. Similar morphology was observed for three independent clones. b, Apoptosis assay in control, K4A, or K36A mutant NPCs on day 8 of neuronal differentiation. Caspase3/7 activated (green fluorescence) apoptotic cells were detected using flow cytometry analysis. P value: unpaired, two-sided Wilcoxon rank sum test; n = 5 independent replicates from two clonal lines per group. c, Gating strategy for cell cycle analysis by flow cytometry (data in Main Fig. 1c). Wild type ESCs were labeled with EdU (proliferating cells) and DAPI (DNA content). Single cells detected by analyzing the DAPI cell area versus the DAPI height fall on the diagonal between the two axes. Intact cells were selected by comparison of cell size (FSC-A) and cell granularity (SSC-A). For detecting the cell cycle phase, EdU signal was compared to DAPI. This gating strategy has been applied to generate the data in Fig. 1c, in which n = 2 or n = 4 independent biological replicates showed similar results. d, Gating strategy for apoptosis analysis by flow cytometry. Control ESCs were labeled with active Caspase-3/7 (apoptotic cells) and propidium iodide (permeable necrotic cells). For detecting necrotic, early apoptotic, and late apoptotic cells, intact cells were selected and Caspase-3/7 signal was compared to propidium iodide signal. e, Representative bright-field images of NPC embryoid bodies (EBs – D4) of controls, K4A and K36A mutants grown in suspension. Experiments have been repeated at least three times independently with similar results. f, Expression of cell adhesion genes in control and K4A cells. Normalized RNA-seq reads of markers annotated in the GO term ‘regulation of cell-cell adhesion’ are shown as points for each of n = 2 (control) or n = 3 (K4A) independent clonal lines.
Extended Data Fig. 4 Cellular differentiation defects in H3.3K4A and H3.3K36A ESCs.
a-b, Some of the most enriched biological GO terms (topGO, Bioconductor) for upregulated genes (log2(FoldChange)>0.58, P adj<0.05) in H3.3K36A (a) or H3.3K4A (b) neurons on day 12. Data are the negative decimal logarithm of the enrichment P value (FDR) derived from a one-sided Fisher’s exact test. c, Heatmap of RNA-seq data displaying gene expression changes during neuronal development of wild type ESCs into glutamatergic neurons. All genes were sorted into five clusters (k-means) according to their expression during neurodevelopment (D0, D4, D8, D12). Most enriched biological GO terms were from DAVID. d, Gene expression changes of H3.3K4A/K36A ESCs (left) and neurons (right) compared to controls were plotted per gene cluster defined in c. Positive and negative values represent the overall higher and lower expression of genes, respectively, compared to controls. Boxplot indicates medians (middle line), third and first quartile (box), and whiskers show 1.5 × the interquartile range above and below the box. e, Normalized RNA-seq counts for neural stem cell markers (Nestin, Notch1, Sox2) and glutamatergic neuron markers (Grin2b, Slc17a6, Grin1) in WT, controls, H3.3K36A, H3.3K4A neurons, and WT neural precursor cells (NPCs – Day 8). P value: Wald test in DESeq2 and adjusted using Benjamini Hochberg’s method, ns: P > 0.05. Samples in a,b,d,e were n = 2 (control), n = 3 (K4A) or n = 3 (K36A) independent clonal lines. f, Representative bright-field images of cells on day 14 of cardiac differentiation. Experiment was repeated three times with similar results. g, Quantification of contracting cardiomyocyte colonies per cell culture dish. The data are mean ± s.d.; two independent clonal lines.
Extended Data Fig. 5 H3.3K4A mutation does not change global histone modification levels, but reduces H3K4me3 levels outside of TSS.
a, Volcano plot displaying global changes in histone modifications in H3.3K4A mutant ESCs compared to controls. Log2(FoldChanges) were calculated for canonical H3.1/H3.2, compared to controls and plotted against -log2(P values). Unpaired, two-sided Student’s t-test. n = 3 biological replicates. The 7 most significant modifications are depicted in black. H3K4me3 was not detected in this analysis. b, Scatter plot correlating genic H3K36me3 levels with gene expression changes in NPCs (D8). Significant Fold-changes (FDR < 0.05) of genic H3K36me3 levels (x-axis) are plotted against log2(FoldChanges) in gene expression (y-axis) measured in H3.3K36A mutants compared to wild type. Green dots indicate significant gene expression changes (P adj < 0.05, absolute log2(FoldChange)>0.58) and numbered per quadrant. Pearson correlation coefficients (P) are depicted in plots. P value: Wald test in DESeq2 and adjusted using Benjamini Hochberg’s method; n = 2 independent replicates. c, Boxplot depicting H3K36me3 levels in alternatively spliced exons in H3.3K36A and control ESCs. H3K36me3-ChIP-seq (RPKM) were calculated for exons that were significantly included or excluded from the mRNA, or for random exons that were not differentially spliced. Numbers of analyzed genomic features (n) are depicted underneath boxplot. P value: unpaired, two-sided Wilcoxon rank sum test; ns: P > 0.05. n = 2 independent replicates. d, Boxplot displaying significant FoldChanges of H3K4me3 signal to the nearest gene in H3.3K4A mutant relative to controls. Wald test in DiffBind/DESeq2 and adjusted using Benjamini Hochberg’s method (FDR < 0.05). Numbers of differential peaks are depicted in box. Boxplots in c-d indicate medians (middle line), third and first quartile (box), and whiskers show 1.5 × the interquartile range above and below the box.
Extended Data Fig. 6 Depletion of H3.3K4A at TSS occurs rapidly and is independent of histone chaperones.
a, Experimental overview for a pulse labeling of H3.3 protein. b, ChIP-qPCR results for H3.3-SNAP Biotin labeling experiment. Relative enrichment over the input of H3.3 wild type, K4A, K36A, and K4R (lysine to arginine) mutants was measured at TSS or TES of indicated genes, or a gene-free region on chromosome 6 (neg. control). H3.3 decrease over time indicates exchange for newly synthesized (unlabeled) histones. The data are mean ± s.d.; n = 2 independent cell lines. c, H3.3 enrichment (RPKM) at genic regions and enhancers in control and HIRA knockout (HIRAnull) ESCs. ChIP-seq data were obtained from Goldberg et al.23 Boxplot indicates medians (middle line), third and first quartile (box) and whiskers show 1.5 × the interquartile range above and below the box. d, Interaction of wild type and mutant H3.3 with histone chaperones Daxx and Atrx. Log2(signal intensities) showed that both were identified to bind mutant and wild type nucleosomes. Boxplot indicates mean (middle line), third and first quartile (box) and whiskers show and minimum and maximum data points (n = 2 independent clonal lines). e, Immunoblot analysis in nuclear lysates of WT, control, K4A and K36A ESCs for protein levels of Atrx and Hira. Canonical H3 (H3.1/H3.2) serves as a loading control. Experiments were repeated three times independently with similar results. f, Normalized RNA-seq counts for gene expression of Atrx/Daxx and Hira chaperone complexes in WT, controls, K36A, K4A neurons. P value: Wald test and adjustment using Benjamini Hochberg’s method in DESeq2, n.s: P adj > 0.05; n = 2 (control), n = 3 (K4A/K36A) or n = 4 (WT) independent biological replicates Source data.
Extended Data Fig. 7 Lysine 4 residue is required for histone H3 protein stability in HEK293T cells.
a, Normalized RNA-seq counts representing H3.3B gene expression in control, K36A, K4A mutant clonal lines, and wild type (WT) in ESCs and neurons. P value: Wald test and adjustment using Benjamini Hochberg’s method in DESeq2, n.s: P adj > 0.05; n = 2 (control), n = 3 (K4A/K36A) or n = 4 (WT) independent biological replicates. Significantly lower H3.3B gene expression in K36A cells is partially due to difficulties in mapping (5 nucleotide changes introduced). b-e, Stability analysis of H3.3 mutants in HEK293T cells. H3.3/H3.1 construct fused to HA-FLAG-tag for detection and co-transcribed with P2A-GFP generates two proteins, mostly nuclear H3.3-HA-FLAG and mostly cytosolic P2A-GFP (expression control). Immunoblots of cellular fractions of HEK293T transfected with WT or K4A/R/Q or K36A/R/Q mutant H3.3 (Q, glutamine and R, arginine) (b) and transfected with WT or K4A/K36A mutant H3.3 (c). Immunoblots of whole-cell lysates of HEK293T transfected with WT and K4A mutant H3.1/H3.3 (d) and chromatin fraction of HEK293T untreated or treated with the proteasome inhibitor MG-132 (5 μM for 5 hours prior to harvesting) (e). Suz12 and endogenous H3.3 serve as a loading control. Experiments in b-e were repeated three times independently with similar results. f, H3.3 ChIP-qPCR results in control and K4A mutant ESCs untreated or treated with the proteasome inhibitor MG-132 (5 μM for 5 hours). Relative enrichment of H3.3 wild type or H3.3K4A over input was measured at TSS or TES of indicated genes, or a gene-free region on chromosome 6 (neg. control). The data are represented as the mean of two independent replicates (n = 2) Source data.
Extended Data Fig. 8 Depletion of NuRD and Swi/Snf remodelers reduces H3.3 occupancy inside regulatory regions and genes in HEK293T cells.
a, Immunoblots of nuclear fractions of HEK293T cells after siRNA-mediated depletion of Chd4, Smarca4, or without a target (negative control). Depletion was confirmed by using specific antibodies against Chd4 or Smarca4, H3.3 antibody is used as control. Experiment was repeated three times independently with similar results. b, Analysis of H3.3 enrichment at indicated genomic regions in HEK293T cells depleted of Chd4 or Smarca4. H3.3-ChIP-seq reads were normalized to library size and input (dashed line) and calculated for the following features: TSSs (±1.5kb), gene body and TESs (±1.5kb) of protein-coding genes. Values higher than those of input (dashed line) indicate enrichment of H3.3. P value: unpaired, two-sided Wilcoxon rank sum test; n = 3 independent replicates. c, Analysis of nascent transcription levels (PRO-seq) in control and H3.3K4A ESCs at H3K4me3 only TSSs, bivalent TSSs (H3K4me3 + H3K27me3) or H3K27me3 only TSSs. Strand-specific PRO-seq (RPKM, shown in log2 scale) were calculated for indicated TSSs (±1.5kb). P value: unpaired, two-sided Wilcoxon rank sum test; n = 2 independent replicates. d, Boxplot displaying H3K4me3 and H3K27me3 levels at TSS (±1.5kb) of H3K4me3 only, bivalent, or H3K27me3 only genes in K4A mutants. RPKM were calculated for TSS (±1.5kb) regions from the ChIP-seq experiment in control and K4A ESCs and are depicted in comparison to input levels. n = 3 independent replicates. Boxplots in b-d indicate medians (middle line), third and first quartile (box) and whiskers show 1.5 × the interquartile range above and below the box Source data.
Extended Data Fig. 9 Chromatin environment at regulatory regions and existing nucleosome dynamics affect gene expression changes in K4A mutant ESCs.
a, Significant GO terms (biological processes) in clusters of DEGs from the heatmap in Fig. 6a. P value: hypergeometric test and adjustment using Benjamini Hochberg’s method; n = 2 (control), n = 3 (K4A) clonal lines. b, Boxplot displaying H3K27ac levels at TSS (±1.5kb) of DEGs and unchanged genes (random) in K4A and K36A mutants. Log2(RPKM) were calculated from ChIP-seq in control, K4A, K36A ESCs, and compared to inputs. n = 2 (control, K36A), n = 3 (K4A) clonal lines. c, HDAC (Hdac1) and HAT (Cbp) enrichment in control, K4A, K36A ESCs at indicated genomic regions. Numbers of genomic features (n) are depicted underneath boxplot. RPKM were normalized to input (dashed line). n = 1 clonal line. d, Boxplot depicting average histone turnover levels in WT background ESCs at TSSs (±1.5kb) of DEGs (FDR<0.05) or unchanged genes from K4A ESCs. e, Boxplot displaying Ser5Phos-RNA Pol II inside genebodies, TSS(+1.5kb) to TES (-1.5kb), of DEGs or unchanged genes (other) in K4A ESCs. Log2(RPKM) were from ChIP-seq in control, K4A, K36A ESCs. n = 2 clonal lines. f, Boxplot depicting average ChIP-seq signal, log2(RPKM), of Brg1, Chd4, H3K27me3 in K4A mutant and control ESCs at weak, medium and strong enhancers separated by PRO-seq counts (same as in Fig. 6d). n = 2 (Chd4, Brg1), n = 3 (H3K27me3) clonal lines. g, Boxplot depicting average histone turnover levels in WT background ESCs at weak, medium and strong enhancers. Data in d, g was taken from Banaszynski et al.12; n = 1 cell line. P value in c-g: unpaired, two-sided Wilcoxon rank sum test; P >0.05 ns. Boxplots in b-g indicate medians (middle line), third and first quartile (box) and whiskers show 1.5 × the interquartile range above and below the box.
Extended Data Fig. 10 Quality control of ChIP-seq data.
a, PCA analysis plots displaying differences/similarities between samples for H3.3-, H3K27ac-, H3K4me1- and H3K4me3-ChIP-Seq experiments. PCA analysis was done on binned and library size normalized bigWig files. H3.3: n = 2, H3K27ac/H3K4me1/H3K4me3: n = 2 (control) or n = 3 (K4A) clonal lines. b, MA-plot displaying logFoldChanges in signal intensities in ChIP-seq data of H3.3K4A mutants compared to controls. Sites that are identified to be significantly differentially (FDR < 0.05) bound are shown in red and were identified using DiffBind package. Total number of significant changes (more or less bound than control) are depicted in each plot. P value: Wald test and adjustment using Benjamini Hochberg’s method in DiffBind/DESeq2; H3.3: n = 2, H3K27ac/H3K4me1/H3K4me3: n = 2 (control) or n = 3 (K4A) clonal lines.
Supplementary information
Supplementary Information
Supplementary Note
Supplementary Tables
Supplementary Tables 1–3
Source data
Source Data Fig. 3
Unprocessed immunoblots with indicated cropping ranges depicted in Fig. 3a.
Source Data Fig. 4
Unprocessed immunoblots with indicated cropping ranges depicted in Fig. 4g.
Source Data Extended Data Fig. 6
Unprocessed immunoblots with indicated cropping ranges depicted in Extended Data Fig. 6e.
Source Data Extended Data Fig. 7
Unprocessed immunoblots with indicated cropping ranges depicted in Extended Data Fig. 7b–e.
Source Data Extended Data Fig. 8
Unprocessed immunoblots with indicated cropping ranges depicted in Extended Data Fig. 8a.
Rights and permissions
About this article
Cite this article
Gehre, M., Bunina, D., Sidoli, S. et al. Lysine 4 of histone H3.3 is required for embryonic stem cell differentiation, histone enrichment at regulatory regions and transcription accuracy. Nat Genet 52, 273–282 (2020). https://doi.org/10.1038/s41588-020-0586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-020-0586-5
This article is cited by
-
HIRA vs. DAXX: the two axes shaping the histone H3.3 landscape
Experimental & Molecular Medicine (2024)
-
H3.3 contributes to chromatin accessibility and transcription factor binding at promoter-proximal regulatory elements in embryonic stem cells
Genome Biology (2023)
-
Dynamic changes in whole genome DNA methylation, chromatin and gene expression during mouse lens differentiation
Epigenetics & Chromatin (2023)
-
Histone exchange sensors reveal variant specific dynamics in mouse embryonic stem cells
Nature Communications (2023)
-
ARID1A-dependent maintenance of H3.3 is required for repressive CHD4-ZMYND8 chromatin interactions at super-enhancers
BMC Biology (2022)