Abstract
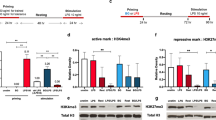
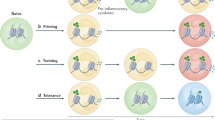
Accumulation of trimethylation of histone H3 at lysine 4 (H3K4me3) on immune-related gene promoters underlies robust transcription during trained immunity. However, the molecular basis for this remains unknown. Here we show three-dimensional chromatin topology enables immune genes to engage in chromosomal contacts with a subset of long noncoding RNAs (lncRNAs) we have defined as immune gene–priming lncRNAs (IPLs). We show that the prototypical IPL, UMLILO, acts in cis to direct the WD repeat-containing protein 5 (WDR5)–mixed lineage leukemia protein 1 (MLL1) complex across the chemokine promoters, facilitating their H3K4me3 epigenetic priming. This mechanism is shared amongst several trained immune genes. Training mediated by β-glucan epigenetically reprograms immune genes by upregulating IPLs in manner dependent on nuclear factor of activated T cells. The murine chemokine topologically associating domain lacks an IPL, and the Cxcl genes are not trained. Strikingly, the insertion of UMLILO into the chemokine topologically associating domain in mouse macrophages resulted in training of Cxcl genes. This provides strong evidence that lncRNA-mediated regulation is central to the establishment of trained immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. RNA-seq data are available in the Gene Expression Omnibus under accession number GSE120621.
Change history
15 January 2019
In the version of this article initially published, ‘+’ and ‘–’ labels were missing from the graph keys at the bottom of Fig. 8d. The error has been corrected in the HTML and PDF versions of the article.
References
Rogatsky, I. & Adelman, K. Preparing the first responders: building the inflammatory transcriptome from the ground up. Mol. Cell 54, 245–254 (2014).
Bhatt, D. M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Lauberth, S. M. et al. H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell 152, 1021–1036 (2013).
Quintin, J. et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 (2012).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, 6284 (2016).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Novakovic, B. et al. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368 (2016).
Li, G. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Fanucchi, S. et al. Chromosomal contact permits transcription between coregulated genes. Cell 155, 606–620 (2013).
Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Jin, F. et al. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature 503, 290–294 (2013).
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Dekker, J. & Mirny, L. The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121 (2016).
Guttman, M. et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477, 295–300 (2011).
Gomez, J. A. et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 152, 743–754 (2013).
Quinodoz, S. & Guttman, M. Long noncoding RNAs: an emerging link between gene regulation and nuclear organization. Trends Cell Biol. 24, 651–663 (2014).
Andersson, R. et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 (2014).
Lai, F. et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 494, 497–501 (2013).
Wang, K. C. et al. A long non-coding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011).
Yang, Y. W. et al. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. eLife 3, e02046 (2014).
Paulsen, M. T. et al. Coordinated regulation of synthesis and stability of RNA during the acute TNF-induced proinflammatory response. Proc. Natl Acad. Sci. USA 110, 2240–2245 (2013).
Brown, J. D. et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol. Cell 56, 219–231 (2014).
Papantonis, A. et al. TNFα signals through specialized factories where responsive coding and miRNA genes are transcribed. EMBO J. 31, 4404–4414 (2012).
Matsushima, K. & Morishita, K. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J. Exp. Med. 167, 1883–1893 (1988).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Wang, X. et al. MLL1, a H3K4 methyltransferase, regulates the TNFα-stimulated activation of genes downstream of NF-κB. J. Cell Sci. 125, 4058–4066 (2012).
Cao, F. et al. Targeting MLL1 H3K4 methyltransferase activity in mixed-lineage leukemia. Mol. Cell 53, 247–261 (2014).
Raj, A. et al. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Shibayama, Y., Fanucchi, S. & Mhlanga, M. M. Visualization of enhancer-derived noncoding RNA. Methods Mol. Biol. 1468, 19–32 (2017).
Engreitz, J. M. et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455 (2017).
Goodridge, H. S. et al. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178, 3107–3115 (2007).
Asfaha, S. et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 144, 155–166 (2013).
Sauter, C. & Wolfensberger, C. Interferon in human serum after injection of endotoxin. Lancet 2, 852–853 (1980).
Thin, L. W. et al. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm. Bowel Dis. 19, 1490–1498 (2013).
Zemach, A., McDaniel, I. E., Silva, P. & Zilberman, D. Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328, 916–919 (2010).
Kærn, M. et al. Stochasticity in gene expression: from theories to phenotypes. Nat. Rev. Genet. 6, 451–464 (2005).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470 (2016).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 10, 89–100 (2018).
Campbell, L. M. et al. Rationale and means to target pro-inflammatory interleukin-8 (CXCL8). Signal. CancerPharmaceut. 6, 929–959 (2013).
Repnik, U., Knezevic, M. & Jeras, M. Simple and cost-effective isolation of monocytes from buffy coats. J. Immunol. Methods 278, 283–292 (2003).
Imakaev, M. et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nat. Methods 9, 999–1003 (2012).
Li, G. et al. ChIA-PET tool for comprehensive chromatin interaction analysis with paired-end tag sequencing. Genome Biol. 11, R22 (2010).
Tennakoon, C. et al. BatMis: a fast algorithm for k-mismatch mapping. Bioinformatics 28, 2122–2128 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Chu, C. & Chang, H. Y. Understanding RNA-chromatin interactions using chromatin isolation by RNA purification (ChIRP). Methods Mol. Biol. 1480, 115–123 (2016).
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Samulski, R. J. et al. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 61, 3096–3101 (1987).
Grimm, D. Production methods for gene transfer vectors based on adeno-associated virus serotypes. Methods 28, 146–157 (2002).
Grimm, D. et al. In vitro and in vivo gene therapy vector evolution via multi species interbreeding and retargeting of adeno-associated viruses. J. Virol. 82, 5887–5911 (2008).
Shevchenko, A. et al. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2007).
Hagege, H. et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protoc. 2, 1722–1733 (2007).
Acknowledgements
We thank all members of the Gene Expression and Biophysics Laboratory (the M.M.M. laboratory). We thank M. Lusic, A Gontijo, F. Brombacher, Y. Negishi, L. Davignon and INTRIM consortium members for comments on the manuscript. The authors also thank S. Consalvi, M. Charpentier, A. Boucharlat and the Chemogenomic and Biological screening core facility at the Institut Pasteur in Paris for support during the course of this work. This research is supported by a Department of Science and Technology Centre of Competence Grant, an SA Medical Research Council SHIP grant, and a CSIR Parliamentary Grant, all to M.M.M., and M.M.M. is a Chan Zuckerberg Investigator of the Chan Zuckerberg Initiative. A full list of the investigators who contributed to the generation of the Blueprint Consortium data used in the ChIP-seq project is available from http://www.blueprint-epigenome.eu. Funding for that project was provided by the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement number 282510–BLUEPRINT.
Author information
Authors and Affiliations
Contributions
S.F. and M.M.M. designed the study. S.F. performed most experiments and collected and analyzed data. E.T.F. carried out 3C experiments and ChIP and analyzed data. E.D. analyzed CAGE, ChIP and RNA-seq data. Y.S. designed 3C experiments and performed RNA FISH experiments. K.B. and D.G. designed and produced the AAV vectors. E.Y.C. and K.C.W. helped design and perform the UMLILO knock-in experiment. S.S. carried out mass spectrometry experiments and analyzed data. M.I. analyzed Hi-C data. G.L. and W.-K.S. analyzed ChIP and ChIA-PET data. S.F., Y.S., M.I., E.T.F. and M.M.M. discussed and edited the paper. S.F. and M.M.M. co-wrote the paper. M.M.M. designed experiments, analyzed data and supervised the study.
Corresponding author
Ethics declarations
Competing interests
CSIR (Pretoria) has filed a provisional patent application on behalf of S.F., Y.S., E.D. and M.M.M. claiming some of the concepts described in this publication and licensed the patent to Immunolincs Genomics (Seattle, WA).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18
Supplementary Table 1
Coordinates and tissue-specific expression of the IPLs
Supplementary Table 2
Chromatin interactions between TNF-responsive genes and lncRNAs in unstimulated HUVECs
Supplementary Table 3
Chromatin interactions between TNF-responsive genes and lncRNAs in HUVECs stimulated with TNF for 30 min
Supplementary Table 4
List of siRNA, LNA and oligonucleotide sequences
Rights and permissions
About this article
Cite this article
Fanucchi, S., Fok, E.T., Dalla, E. et al. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat Genet 51, 138–150 (2019). https://doi.org/10.1038/s41588-018-0298-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-018-0298-2
This article is cited by
-
Disparate macrophage responses are linked to infection outcome of Hantan virus in humans or rodents
Nature Communications (2024)
-
A chromatin-regulated biphasic circuit coordinates IL-1β-mediated inflammation
Nature Genetics (2024)
-
Trained immunity in atherosclerotic cardiovascular disease
Nature Reviews Cardiology (2023)
-
A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis
Cell Research (2023)
-
Remembering foods and foes: emerging principles of transcriptional memory
Cell Death & Differentiation (2023)