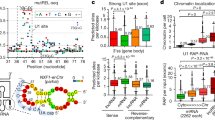
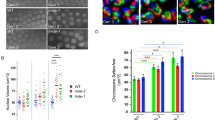
Abstract
SCHLAP1 is a long noncoding RNA that is reported to function by depleting the SWI/SNF complex from the genome. We investigated the hypothesis that SCHLAP1 affects only specific compositions of SWI/SNF. Using several assays, we found that SWI/SNF is not depleted from the genome by SCHLAP1 and that SWI/SNF is associated with many coding and noncoding RNAs, suggesting that SCHLAP1 may function in a SWI/SNF-independent manner.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Prensner, J. R. et al. Nat. Genet. 45, 1392–1398 (2013).
Prensner, J. R. et al. Lancet Oncol. 15, 1469–1480 (2014).
Raab, J. R., Resnick, S. & Magnuson, T. PLoS Genet. 11, e1005748 (2015).
Raab, J. R., Runge, J. S., Spear, C. C. & Magnuson, T. Epigenetics Chromatin 10, 62 (2017).
Kadoch, C. & Crabtree, G. R. Cell 153, 71–85 (2013).
Kuwahara, Y., Wei, D., Durand, J. & Weissman, B. E. Mol. Cancer Res. 11, 251–260 (2013).
Morris, S. A. et al. Nat. Struct. Mol. Biol. 21, 73–81 (2014).
Nakayama, R. T. et al. Nat. Genet. 49, 1613–1623 (2017).
Luo, Z., Rhie, S. K., Lay, F. D. & Farnham, P. Cell Rep. 21, 1411–1417 (2017).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Nat. Methods 10, 1213–1218 (2013).
Zanconato, F. et al. Nat. Cell Biol. 17, 1218–1227 (2015).
Cheng, S., Wang, L., Deng, C.-H., Du, S.-C. & Han, Z.-G. Biochem. Biophys. Res. Commun. 491, 178–182 (2017).
Kawaguchi, T. et al. Proc. Natl Acad. Sci. USA 112, 4304–4309 (2015).
Wang, Y. et al. Cell Stem Cell 16, 413–425 (2015).
Zhu, Y., Rowley, M. J., Böhmdorfer, G. & Wierzbicki, A. T. Mol. Cell 49, 298–309 (2013).
Cajigas, I. et al. Development 142, 2641–2652 (2015).
Lino Cardenas, C. L. et al. Nat. Commun. 9, 1009 (2018).
Hendrickson, D. G., Kelley, D. R., Tenen, D., Bernstein, B. & Rinn, J. L. Genome. Biol. 17, 28 (2016).
Clemson, C. M. et al. Mol. Cell 33, 717–726 (2009).
Hirose, T. et al. Mol. Biol. Cell. 25, 169–183 (2014).
Naganuma, T. et al. EMBO J. 31, 4020–4034 (2012).
Kaneko, S., Son, J., Shen, S. S., Reinberg, D. & Bonasio, R. Nat. Struct. Mol. Biol. 20, 1258–1264 (2013).
Méndez, J. & Stillman, B. Mol. Cell. Biol. 20, 8602–8612 (2000).
Herrmann, C., Avgousti, D. C. & Weitzman, M. D. Bio. Protoc. 7, e2175 (2017).
Langmead, B. & Salzberg, S. L. Nat. Methods 9, 357–359 (2012).
Li, H. et al. Bioinformatics 25, 2078–2079 (2009).
Ramírez, F. et al. Nucleic Acids Res. 44, W160–W165 (2016).
Robinson, J. T. et al. Nat. Biotechnol. 29, 24 (2011).
Zhang, Y. et al. Genome. Biol. 9, R137 (2008).
Zhu, L. J. et al. BMC Bioinformatics 11, 237 (2010).
Lun, A. T. L. & Smyth, G. K. Nucleic Acids Res. 44, e45 (2016).
Heinz, S. et al. Mol. Cell 38, 576–589 (2010).
Acknowledgements
We thank members of the Magnuson laboratory for helpful comments and discussion. We thank B. Weissman for the G401;SNF5 inducible cell line and A. Chinnaiyan for the SCHLAP1 expression construct. This work was supported by grants to J.R.R. from The University of North Carolina at Chapel Hill University Cancer Research Fund (Tier 1 Development Award) and North Carolina Translational and Clinical Sciences Institute (2KR851610). In addition, this work was supported by grants from The National Institute of Child Health and Development (5R01HD036655 to T.M.) and The National Institute of General Medical Sciences (5F32GM108367 to J.R.R. and R01GM121806 to J.M.C.).
Author information
Authors and Affiliations
Contributions
J.R.R. and T.M. conceived the study. J.R.R., K.N.S., and J.M.C. developed the methodology. J.R.R., K.N.S., C.J.M., and C.C.S. performed the investigation. J.R.R. performed data curation. J.R.R. and T.M. wrote the original draft. J.R.R., K.N.S., and T.M. contributed to manuscript review and editing. J.R.R. and T.M. were involved in funding acquisition. J.R.R. and T.M. supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 SCHLAP1 has minimal effects on SWI/SNF composition and cell growth.
a, Western blot of SWI/SNF subunits in RWPE1;LACZ and RWPE1;SCHLAP1 cells. The experiment was repeated with two independent cell lines. b, Growth of RWPE1;LACZ and RWPE1;SCHLAP1 cells measured in 2D growth assay. The experiment was performed on three independent cell lines. Error bars represent mean and s.d. c, Growth assay performed on cell lines in b starting from 10 or 100 cells per well in 96-well low-adherent plates. The experiment was performed using one cell line by seeding 16 independent wells with two concentrations of cells. Boxplots show first, second, and third quartile, and whiskers extend to 1.5 times the interquartile range.
Supplementary Figure 2 Expression of SWI/SNF subunits in cell lines expressing SCHLAP1.
Full western blots for the analysis presented in Supplementary Fig. 1a.
Supplementary Figure 3 SCHLAP1 does not affect SWI/SNF composition or localization.
a, Salt fractionation of RWPE1;LACZ and RWPE1;SCHLAP1 cells for SWI/SNF subunits from whole cells. The experiment was repeated twice with similar results. C, cytosol; N, nucleus; M, micrococcal nuclease digestion. Numbers denote salt fraction (mM). b, Immunofluorescence of RWPE1;LACZ or RWPE1;SCHLAP1 cells for SMARCB1 or SMARCA4. The experiment was repeated twice with similar results. c, Co-immunoprecipitation for SMARCA4 and western blot for other SWI/SNF subunits. The experiment was repeated twice with similar results.
Supplementary Figure 4 SWI/SNF associates with chromatin in the presence of SCHLAP1.
Full western blots for Fig. 1d.
Supplementary Figure 5 SWI/SNF remains associated with chromatin at high salt concentrations in the presence of SCHLAP1.
Full western blots for Supplementary Fig. 3a.
Supplementary Figure 6 SWI/SNF complex remains assembled in the presence of SCHLAP1.
Full western blots for Supplementary Fig. 3c.
Supplementary Figure 7 SCHLAP1 does not disrupt SMARCB1 from arresting G401 cells.
a, Western blot showing doxycycline induction of SMARCB1 in G401;LACZ or G401;SCHLAP1 cells. b, Representative images of crystal violet–stained G401 cells. Two independent cell lines of each genotype were induced and plated for quantification in duplicate.
Supplementary Figure 8 SNF5 is induced by doxycycline in G401 cells.
Full western blot for Supplementary Fig. 7a.
Supplementary Figure 9 SWI/SNF occupancy in the presence of SCHLAP1.
a, Overlap between SMARCA2 and SMARCA4 peaks. b, Genomic features associated with SWI/SNF peaks.
Supplementary Figure 10 SCHLAP1 induces open chromatin changes but does not alter histone modifications.
a, Example loci representing open and closed chromatin regions from three independent replicate ATAC-seq experiments on two independent SCHLAP1 cell lines. b, Genomic loci associated with open, closed, and static sites. *P = 0.001, chi-squared test. c, The heatmap displays open chromatin, H3K4me3, H3K4me1, or H3K27ac signal in RWPE1;LACZ or RWPE;SCHLAP1 cells. Rows are ordered according to level of open chromatin signal. d, The heatmap shows the level of three SWI/SNF subunits present in RWPE1;SCHLAP1 cells at altered open chromatin sites. Order is the same as in c.
Supplementary Figure 11 SCHLAP1-induced chromatin changes are associated with specific processes and motifs.
a, Enrichment analysis of sites that either open or close following SCHLAP1 expression in RWPE1 cells from three independent ATAC-seq experiments on two independent SCHLAP1 cell lines. Enrichment analysis was performed using HOMER software32. b, Motif analysis showing enriched motifs in open compared to closed, or closed compared to open, chromatin.
Supplementary Figure 12 SCHLAP1-induced chromatin changes do not cause concordant gene expression changes.
Significance and changes in expression of gene expression microarrays performed by Prensner et al.1 on RWPE1;LACZ or RWPE1;SCHLAP1 cells compared to the altered chromatin regions. Each panel represents the set of regions that open, close, or remain static in our ATAC-seq assay. b, Quantification of the number of genes that are significantly differentially expressed and that are associated with an ATAC-seq peak. These represent those genes that fall below the adjusted P-value threshold of 0.05 in a. P values are from gene expression changes from GSE40385, reanalyzed using the limma package, with no fold change cutoff and adjusted P < 0.05 to identify significant genes.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12
Supplementary Table 1
ChIP-seq peak data
Supplementary Table 2
ATAC-seq peak data
Supplementary Table 3
Motif analysis–upregulated
Supplementary Table 4
Motif analysis–downregulated
Supplementary Table 5
RIP counts 22Rv1
Supplementary Table 6
RIP counts LNCaP
Supplementary Table 7
NGS quality control data
Rights and permissions
About this article
Cite this article
Raab, J.R., Smith, K.N., Spear, C.C. et al. SWI/SNF remains localized to chromatin in the presence of SCHLAP1. Nat Genet 51, 26–29 (2019). https://doi.org/10.1038/s41588-018-0272-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-018-0272-z
This article is cited by
-
Long noncoding RNAs in cancer metastasis
Nature Reviews Cancer (2021)
-
SChLAP1 promotes prostate cancer development through interacting with EZH2 to mediate promoter methylation modification of multiple miRNAs of chromosome 5 with a DNMT3a-feedback loop
Cell Death & Disease (2021)
-
Long non-coding RNAs: the tentacles of chromatin remodeler complexes
Cellular and Molecular Life Sciences (2021)
-
Role of specialized composition of SWI/SNF complexes in prostate cancer lineage plasticity
Nature Communications (2020)
-
LSH interacts with and stabilizes GINS4 transcript that promotes tumourigenesis in non-small cell lung cancer
Journal of Experimental & Clinical Cancer Research (2019)