Abstract
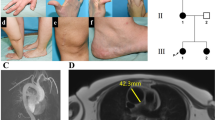
Primary aldosteronism is the most common and curable form of secondary arterial hypertension. We performed whole-exome sequencing in patients with early-onset primary aldosteronism and identified a de novo heterozygous c.71G>A/p.Gly24Asp mutation in the CLCN2 gene, encoding the voltage-gated ClC-2 chloride channel1, in a patient diagnosed at 9 years of age. Patch-clamp analysis of glomerulosa cells of mouse adrenal gland slices showed hyperpolarization-activated Cl– currents that were abolished in Clcn2−/− mice. The p.Gly24Asp variant, located in a well-conserved ‘inactivation domain’2,3, abolished the voltage- and time-dependent gating of ClC-2 and strongly increased Cl– conductance at resting potentials. Expression of ClC-2Asp24 in adrenocortical cells increased expression of aldosterone synthase and aldosterone production. Our data indicate that CLCN2 mutations cause primary aldosteronism. They highlight the important role of chloride in aldosterone biosynthesis and identify ClC-2 as the foremost chloride conductor of resting glomerulosa cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thiemann, A., Gründer, S., Pusch, M. & Jentsch, T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356, 57–60 (1992).
Jordt, S. E. & Jentsch, T. J. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 16, 1582–1592 (1997).
Gründer, S., Thiemann, A., Pusch, M. & Jentsch, T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature 360, 759–762 (1992).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 389, 37–55 (2017).
Hannemann, A. & Wallaschofski, H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies—a review of the current literature. Horm. Metab. Res. 44, 157–162 (2012).
Calhoun, D. A. Hyperaldosteronism as a common cause of resistant hypertension. Annu. Rev. Med. 64, 233–247 (2013).
Zennaro, M. C., Boulkroun, S. & Fernandes-Rosa, F. Genetic causes of functional adrenocortical adenomas. Endocr. Rev. 38, 516–537 (2017).
Funder, J. W. et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 101, 1889–1916 (2016).
Savard, S., Amar, L., Plouin, P. F. & Steichen, O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 62, 331–336 (2013).
Rossi, G. P. et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 48, 2293–2300 (2006).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Azizan, E. A. et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 45, 1055–1060 (2013).
Scholl, U. I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 45, 1050–1054 (2013).
Scholl, U. I. et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife 4, e06315 (2015).
Daniil, G. et al. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine 13, 225–236 (2016).
Beuschlein, F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 45, 440–444 (2013).
Lifton, R. P. et al. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355, 262–265 (1992).
Moss, S. J. & Smart, T. G. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2, 240–250 (2001).
Rinke, I., Artmann, J. & Stein, V. ClC-2 voltage-gated channels constitute part of the background conductance and assist chloride extrusion. J. Neurosci. 30, 4776–4786 (2010).
Koch, M. C. et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 257, 797–800 (1992).
Guo, J. H. et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat. Commun. 5, 4420 (2014).
Chorvátová, A., Gendron, L., Bilodeau, L., Gallo-Payet, N. & Payet, M. D. A Ras-dependent chloride current activated by adrenocorticotropin in rat adrenal zona glomerulosa cells. Endocrinology 141, 684–692 (2000).
Jentsch, T. J. Discovery of CLC transport proteins: cloning, structure, function and pathophysiology. J. Physiol. 593, 4091–4109 (2015).
Bösl, M. R. et al. Male germ cells and photoreceptors, both dependent on close cell–cell interactions, degenerate upon ClC-2 Cl– channel disruption. EMBO J. 20, 1289–1299 (2001).
Blanz, J. et al. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J. Neurosci. 27, 6581–6589 (2007).
Hoegg-Beiler, M. B. et al. Disrupting MLC1 and GlialCAM and ClC-2 interactions in leukodystrophy entails glial chloride channel dysfunction. Nat. Commun. 5, 3475 (2014).
Depienne, C. et al. Brain white matter oedema due to ClC-2 chloride channel deficiency: an observational analytical study. Lancet Neurol. 12, 659–668 (2013).
Di Bella, D. et al. Subclinical leukodystrophy and infertility in a man with a novel homozygous CLCN2 mutation. Neurology 83, 1217–1218 (2014).
Boulkroun, S. et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension 59, 592–598 (2012).
Spät, A. & Hunyady, L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol. Rev. 84, 489–539 (2004).
Varela, D., Niemeyer, M. I., Cid, L. P. & Sepúlveda, F. V. Effect of an N-terminus deletion on voltage-dependent gating of the ClC-2 chloride channel. J. Physiol. 544, 363–372 (2002).
Pusch, M., Jordt, S. E., Stein, V. & Jentsch, T. J. Chloride dependence of hyperpolarization-activated chloride channel gates. J. Physiol. 515, 341–353 (1999).
Barrett, P. Q. et al. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J. Physiol. 594, 5851–5860 (2016).
Perez-Reyes, E., Van Deusen, A. L. & Vitko, I. Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. J. Pharmacol. Exp. Ther. 328, 621–627 (2009).
Paul, J. et al. Alterations in the cytoplasmic domain of CLCN2 result in altered gating kinetics. Cell. Physiol. Biochem. 20, 441–454 (2007).
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002).
Hubert, E. L. et al. Mineralocorticoid receptor mutations and a severe recessive pseudohypoaldosteronism type 1. J. Am. Soc. Nephrol. 22, 1997–2003 (2011).
Günther, W., Lüchow, A., Cluzeaud, F., Vandewalle, A. & Jentsch, T. J. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl. Acad. Sci. USA 95, 8075–8080 (1998).
Gomez-Sanchez, C. E. et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell. Endocrinol. 383, 111–117 (2014).
Hu, C., Rusin, C. G., Tan, Z., Guagliardo, N. A. & Barrett, P. Q. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J. Clin. Invest. 122, 2046–2053 (2012).
Wang, T. et al. Comparison of aldosterone production among human adrenocortical cell lines. Horm. Metab. Res. 44, 245–250 (2012).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Gomez-Sanchez, C. E. et al. The production of monoclonal antibodies against aldosterone. Steroids 49, 581–587 (1987).
Acknowledgements
This work was funded through institutional support from INSERM and by the Agence Nationale pour la Recherche (ANR-13-ISV1-0006-01), the Fondation pour la Recherche Médicale (DEQ20140329556), the Programme Hospitalier de Recherche Clinique (PHRC grant AOM 06179), and institutional grants from INSERM. The laboratory of M.-C.Z. is also a partner of the H2020 project ENSAT-HT grant number 633983. T.J.J. was supported by institutional funding from the Leibniz and Helmholtz associations, a grant from the BMBF (E-RARE 01GM1403), and the Prix Louis-Jeantet de Médecine.
Author information
Authors and Affiliations
Contributions
M.-C.Z., F.L.F.-R., G.D., I.J.O., and T.J.J. designed experiments and wrote the manuscript. T.M.S., M.-C.Z., T.S., and F.L.F.-R. performed and analyzed whole-exome sequencing. M.-C.Z., F.L.F.-R., G.D., R.E.Z., and S.B. performed and analyzed the results of in vitro studies on H295R-S2 cells. I.J.O. performed electrophysiological studies for which the data were analyzed by I.J.O. and T.J.J. C.G. characterized adrenal glands from wild-type and Clcn2−/− mice and performed western blots. V.J., X.J., L.A., and H.L. were responsible for patient recruitment, medical care, and clinical data acquisition. All authors revised the manuscript draft. C.G. and R.E.Z. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Dependence of ClC-2WT and ClC-2Asp24 currents on external pH.
WT and mutant channels were expressed in Xenopus oocytes and measured by two-electrode voltage-clamp using a pulse protocol that clamped the oocytes in 2-s-long 20-mV steps from +60 to –120 mV. a,b, Representative current traces obtained from WT (a) and G24D mutant (b) ClC-2 at indicated pH values. c,d, Mean ClC-2WT (c) and ClC-2Asp24 (d) currents measured after 2 s as a function of voltage and pH. n = 3–6 oocytes; error bars, s.e.m. (e) Currents at –80 mV (approximately the resting voltage of glomerulosa cells) from ClC-2WT (filled circles) and ClC-2Asp24 (open circles) normalized to respective currents at –120 mV at pH 7.4. Note the large pH dependence of WT currents, which is strongly reduced but not abolished by the Gly24Asp mutation.
Supplementary Figure 2 Effect of ClC-2 downregulation on aldosterone production and expression of genes involved in aldosterone biosynthesis.
a, Basal and stimulated (Ang II or K+) mRNA expression of CLCN2 in H295R-S2 cells infected with scrambled (open bars) or ClC-2 (filled bars) shRNA (one-way ANOVA, P < 0.0001, F = 28.11). b, Basal and stimulated aldosterone production by H295R-S2 cells infected with scrambled or ClC-2 shRNA. c–e, Basal and stimulated mRNA expression of CYP11B2 (one-way ANOVA, P < 0.0001, F = 84) (c), STAR (Kruskal–Wallis, P = 0.0022) (d), and CYP21A2 (Kruskal–Wallis, P = 0.0002) (e) in H295R-S2 cells transfected with scrambled or ClC-2 shRNA. Results of mRNA expression are represented as fold induction of cells infected with scrambled shRNA in basal conditions. Values of all experiments are represented as means ± s.e.m. of two independent experiments performed in experimental triplicate for each condition (n = 6 for scrambled shRNA, n = 12 for ClC-2 shRNA). *P < 0.05; ***P < 0.001; (i) P < 0.05 stimulated versus basal condition; (ii) P < 0.01 stimulated versus basal condition; (iii) P < 0.001 stimulated versus basal condition.
Supplementary Figure 3 CLCN2 variants identified in subjects with bilateral adrenal hyperplasia.
a, Sanger sequencing chromatograms showing the CLCN2 wild-type sequence and the CLCN2 variant c.143C>G (p.Pro48Arg) identified in subject K963-1 with bilateral adrenal hyperplasia. b, Sanger sequencing chromatograms showing the CLCN2 wild-type sequence and the CLCN2 variant c.197G>A (p.Arg66Gln) identified in subject K1044-1 with bilateral adrenal hyperplasia. c, Alignment and conservation of residues encoded by ClC-2 orthologs. Residues that are conserved among more than three sequences are highlighted in yellow.
Supplementary Figure 4 Electrophysiological analyses of ClC-2Gln66 and ClC-2Arg48 channels.
a–c, Representative chloride current traces measured by two-electrode voltage-clamp from Xenopus oocytes injected with 9.2 ng of human ClC-2WT (a), ClC-2Gln66 (b), or ClC-2Arg48 (c) cRNA. d, Mean ± s.e.m. currents measured after 2 s from experiments in a–c plotted as a function of clamp voltage. The number of cells, obtained from two different batches of oocytes, is indicated in parentheses. e, Summary of Cl– currents at –80 mV and after 2 s for a–c. Statistical analyses for ClC-2Gln66 and ClC-2Arg48 were performed by comparison with ClC-2WT, Mann–Whitney test.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1–3
Rights and permissions
About this article
Cite this article
Fernandes-Rosa, F.L., Daniil, G., Orozco, I.J. et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet 50, 355–361 (2018). https://doi.org/10.1038/s41588-018-0053-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-018-0053-8
This article is cited by
-
The ex vivo perfused mouse adrenal gland—a new model to study aldosterone secretion
Pflügers Archiv - European Journal of Physiology (2024)
-
Primary aldosteronism: molecular medicine meets public health
Nature Reviews Nephrology (2023)
-
KCNJ5 mutation is a predictor for recovery of endothelial function after adrenalectomy in patients with aldosterone-producing adenoma
Hypertension Research (2023)
-
Cryo-EM structures of ClC-2 chloride channel reveal the blocking mechanism of its specific inhibitor AK-42
Nature Communications (2023)
-
Biallelic CLCN2 mutations cause retinal degeneration by impairing retinal pigment epithelium phagocytosis and chloride channel function
Human Genetics (2023)