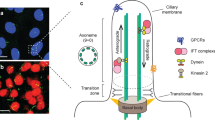
Abstract
Most monogenic cases of obesity in humans have been linked to mutations in genes encoding members of the leptin–melanocortin pathway. Specifically, mutations in MC4R, the melanocortin-4 receptor gene, account for 3–5% of all severe obesity cases in humans1,2,3. Recently, ADCY3 (adenylyl cyclase 3) gene mutations have been implicated in obesity4,5. ADCY3 localizes to the primary cilia of neurons6, organelles that function as hubs for select signaling pathways. Mutations that disrupt the functions of primary cilia cause ciliopathies, rare recessive pleiotropic diseases in which obesity is a cardinal manifestation7. We demonstrate that MC4R colocalizes with ADCY3 at the primary cilia of a subset of hypothalamic neurons, that obesity-associated MC4R mutations impair ciliary localization and that inhibition of adenylyl cyclase signaling at the primary cilia of these neurons increases body weight. These data suggest that impaired signaling from the primary cilia of MC4R neurons is a common pathway underlying genetic causes of obesity in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lubrano-Berthelier, C. et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J. Clin. Endocrinol. Metab. 91, 1811–1818 (2006).
Vaisse, C. et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Invest. 106, 253–262 (2000).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 20, 113–114 (1998).
Stergiakouli, E. et al. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity (Silver Spring) 22, 2252–2259 (2014).
Wang, Z. et al. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One 4, e6979 (2009).
Bishop, G. A., Berbari, N. F., Lewis, J. & Mykytyn, K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 505, 562–571 (2007).
Reiter, J. F. & Leroux, M. R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547 (2017).
Goetz, S. C. & Anderson, K. V. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344 (2010).
Green, J. A. & Mykytyn, K. Neuronal ciliary signaling in homeostasis and disease. Cell. Mol. Life Sci. 67, 3287–3297 (2010).
Hildebrandt, F., Benzing, T. & Katsanis, N. Ciliopathies. N. Engl. J. Med. 364, 1533–1543 (2011).
Vaisse, C., Reiter, J. F. & Berbari, N. F. Cilia and obesity. Cold Spring Harb. Perspect. Biol. 9, a028217 (2017).
Shalata, A. et al. Morbid obesity resulting from inactivation of the ciliary protein CEP19 in humans and mice. Am. J. Hum. Genet. 93, 1061–1071 (2013).
Acs, P. et al. A novel form of ciliopathy underlies hyperphagia and obesity in Ankrd26 knockout mice. Brain Struct. Funct. 220, 1511–1528 (2015).
Davenport, J. R. et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586–1594 (2007).
Wu, L., Shen, C., Seed Ahmed, M., Östenson, C. G. & Gu, H. F. Adenylate cyclase 3: a new target for anti-obesity drug development. Obes. Rev. 17, 907–914 (2016).
Krashes, M. J., Lowell, B. B. & Garfield, A. S. Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci. 19, 206–219 (2016).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Morton, G. J., Meek, T. H. & Schwartz, M. W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15, 367–378 (2014).
Lubrano-Berthelier, C. et al. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum. Mol. Genet. 12, 145–153 (2003).
Aanstad, P. et al. The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr. Biol. 19, 1034–1039 (2009).
Corbit, K. C. et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Bromberg, Y., Overton, J., Vaisse, C., Leibel, R. L. & Rost, B. In silico mutagenesis: a case study of the melanocortin 4 receptor. FASEB J. 23, 3059–3069 (2009).
Calton, M. et al. Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor mutations with severe adult obesity in a large North-American case control study. Hum. Mol. Genet. 18, 1140–1147 (2009).
Ersoy, B. A. et al. Mechanism of N-terminal modulation of activity at the melanocortin-4 receptor GPCR. Nat. Chem. Biol. 8, 725–730 (2012).
Hinney, A. et al. Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J. Clin. Endocrinol. Metab. 88, 4258–4267 (2003).
Berbari, N. F., Johnson, A. D., Lewis, J. S., Askwith, C. C. & Mykytyn, K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell 19, 1540–1547 (2008).
Marley, A., Choy, R. W. & von Zastrow, M. GPR88 reveals a discrete function of primary cilia as selective insulators of GPCR cross-talk. PLoS One 8, e70857 (2013).
Fenselau, H. et al. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci. 20, 42–51 (2017).
Acknowledgements
This research was supported by funds from UCSF DRC NIH P30DK063720 and UCSF NORC NIH P30DK098722; an AMC Graduate School PhD Scholarship to J.E.S.; NIH R01AR05439 and NIH R01GM095941, the Burroughs Wellcome Fund and the David & Lucile F. Packard Foundation to J.F.R.; NIH R01DK60540 to C.V.; NIH RO1DK106404 to C.V. and J.F.R.; NIH RO1DA012864 and NIH RO1DA010711 to M.V.Z.; and a New Frontier Research Award through the UCSF Program for Breakthrough Biomedical Research to C.V., J.F.R. and M.V.Z. We thank K. Deisseroth (Stanford University) for providing plasmids.
Author information
Authors and Affiliations
Contributions
C.V. and J.F.R. supervised the research. J.E.S., Y.W., C.V. and J.F.R. conceived and designed experiments, performed experiments, performed statistical analysis, analyzed the data and wrote the paper. S.Z. performed experiments. A.A.B. performed experiments and analyzed data relevant to Fig. 4. B.A.E. conceived and performed experiments and analyzed data relevant to Fig. 2. A.M. and M.V.Z. contributed regents and expertise relevant to Fig. 4.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Sim1-expressing neurons in the paraventricular nucleus are ciliated
a Hypothalamic PVN section of a Tg(Sim1-EGFP) mouse. Sim1 lineage neurons are in green, the neuronal cilia marker Adcy3 is labeled in red, nuclei in pink. b Increased magnification indicating that all Sim1-expressing neurons possess an Adcy3-positive cilium. c Maximal intensity Z projection of a confocal image of a single EGFP-expressing Sim1 neuron in the PVN. Scale bars represent 10 µm.
Supplementary Figure 2 Serpentine plot of human MC4R
Mutations found in obese patients are indicated by a black arrow from the mutated amino-acid to the colored circle that indicates the amino-acid in the mutant protein. Red arrows indicate frameshift mutations. Most patients were heterozygous for one mutation. Amino-acid changes causing severe effects on MC4R membrane expression are in red. Such mutations are expected to alter membrane and cilia localization as well as ligand response. Mutations in blue lead to decreased agonist activation (blue) or decreased constitutive activity (purple). Whether these mutations also alter cilia localization has not been tested. Mutations tested in Fig. 3a are in yellow. Mutations leading specifically to decreased ciliary expression are indicated by a green arrow.
Supplementary Figure 3 Experimental design and validation of coexpression of GPR88* and mCherry
a, b Design of DIO AAV-expressing Flag-GPR88* or mCherry, stereotaxically injected in experimental a and control b mice. c Coronal section of the PVN of the hypothalamus of Sim1-Cre mice injected with the AAV DIO Flag-GPR88* + AAV DIO mCherry allows for verification of the accuracy and PVN coverage of the injections. d–e Maximal intensity projections of confocal sections through the PVN region indicated by a red square in c show that all mCherry expressing neurons also express Flag-GPR88* (green). White scale bars represent 10 µm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Note and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Siljee, J.E., Wang, Y., Bernard, A.A. et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet 50, 180–185 (2018). https://doi.org/10.1038/s41588-017-0020-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-017-0020-9
This article is cited by
-
Emerging mechanistic understanding of cilia function in cellular signalling
Nature Reviews Molecular Cell Biology (2024)
-
The bi-directional association between bipolar disorder and obesity: Evidence from Meta and bioinformatics analysis
International Journal of Obesity (2023)
-
Genetics and Epigenetics in Obesity: What Do We Know so Far?
Current Obesity Reports (2023)
-
Inferring differential subcellular localisation in comparative spatial proteomics using BANDLE
Nature Communications (2022)
-
The genetics of obesity: from discovery to biology
Nature Reviews Genetics (2022)