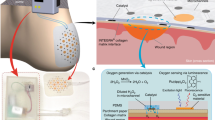
Abstract
‘Smart’ bandages based on multimodal wearable devices could enable real-time physiological monitoring and active intervention to promote healing of chronic wounds. However, there has been limited development in incorporation of both sensors and stimulators for the current smart bandage technologies. Additionally, while adhesive electrodes are essential for robust signal transduction, detachment of existing adhesive dressings can lead to secondary damage to delicate wound tissues without switchable adhesion. Here we overcome these issues by developing a flexible bioelectronic system consisting of wirelessly powered, closed-loop sensing and stimulation circuits with skin-interfacing hydrogel electrodes capable of on-demand adhesion and detachment. In mice, we demonstrate that our wound care system can continuously monitor skin impedance and temperature and deliver electrical stimulation in response to the wound environment. Across preclinical wound models, the treatment group healed ~25% more rapidly and with ~50% enhancement in dermal remodeling compared with control. Further, we observed activation of proregenerative genes in monocyte and macrophage cell populations, which may enhance tissue regeneration, neovascularization and dermal recovery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information.
References
Han, G. & Ceilley, R. Chronic wound healing: a review of current management and treatments. Adv. Ther. 34, 599–610 (2017).
Werdin, F., Tenenhaus, M. & Rennekampff, H.-O. Chronic wound care. Lancet 372, 1860–1862 (2008).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Martin, P. Wound healing–aiming for perfect skin regeneration. Science 276, 75–81 (1997).
Singer, A. J. & Clark, R. A. Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 (1999).
Frykberg, R. G. & Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 4, 560–582 (2015).
Rodrigues, M., Kosaric, N., Bonham, C. A. & Gurtner, G. C. Wound healing: a cellular perspective. Physiol. Rev. 99, 665–706 (2019).
McLister, A., McHugh, J., Cundell, J. & Davis, J. New developments in smart bandage technologies for wound diagnostics. Adv. Mater. 28, 5732–5737 (2016).
Derakhshandeh, H., Kashaf, S. S., Aghabaglou, F., Ghanavati, I. O. & Tamayol, A. Smart bandages: the future of wound care. Trends Biotechnol. 36, 1259–1274 (2018).
Long, Y. et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano 12, 12533–12540 (2018).
Liu, A. et al. Accelerated complete human skin architecture restoration after wounding by nanogenerator-driven electrostimulation. J. Nanobiotechnol. 19, 280 (2021).
Farahani, M. & Shafiee, A. Wound healing: from passive to smart dressings. Adv. Healthc. Mater. 10, e2100477 (2021).
Dincer, C. et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 31, e1806739 (2019).
Barros Almeida, I. et al. Smart dressings for wound healing: a review. Adv. Skin Wound Care 34, 1–8 (2021).
Kekonen, A. et al. Bioimpedance sensor array for long-term monitoring of wound healing from beneath the primary dressings and controlled formation of H2O2 using low-intensity direct current. Sensors 19, 2505 (2019).
Lukaski, H. C. & Moore, M. Bioelectrical impedance assessment of wound healing. J. Diabetes Sci. Technol. 6, 209–212 (2012).
Chanmugam, A. et al. Relative temperature maximum in wound infection and inflammation as compared with a control subject using long-wave infrared thermography. Adv. Skin Wound Care 30, 406–414 (2017).
Tamayol, A. et al. Flexible pH-sensing hydrogel fibers for epidermal applications. Adv. Healthc. Mater. 5, 711–719 (2016).
Xu, G. et al. Battery‐free and wireless smart wound dressing for wound infection monitoring and electrically controlled on‐demand drug delivery. Adv. Funct. Mater. 31, 2100852 (2021).
Trung, T. Q., Ramasundaram, S., Hwang, B. U. & Lee, N. E. An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv. Mater. 28, 502–509 (2016).
Hattori, Y. et al. Multifunctional skin-like electronics for quantitative, clinical monitoring of cutaneous wound healing. Adv. Healthc. Mater. 3, 1597–1607 (2014).
Shi, X. & Wu, P. A smart patch with on-demand detachable adhesion for bioelectronics. Small 17, e2101220 (2021).
Pang, Q. et al. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv. Sci. 7, 1902673 (2020).
Marks, H. et al. A paintable phosphorescent bandage for postoperative tissue oxygen assessment in DIEP flap reconstruction. Sci. Adv. 6, eabd1061 (2020).
Swisher, S. L. et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 6, 6575 (2015).
McCaffrey, C., Flak, J., Kiri, K. & Pursula, P. Flexible bioimpedance spectroscopy system for wound care monitoring. In 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE, 2019).
Kalidasan, V. et al. Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nat. Biomed. Eng. 5, 1217–1227 (2021).
Zhao, Y. et al. Skin‐Inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv. Funct. Mater. 29, 1901474 (2019).
Ciani, I. et al. Development of immunosensors for direct detection of three wound infection biomarkers at point of care using electrochemical impedance spectroscopy. Biosens. Bioelectron. 31, 413–418 (2012).
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021).
Thakral, G. et al. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 4, 22081 (2013).
Kloth, L. C. Electrical stimulation technologies for wound healing. Adv. Wound Care 3, 81–90 (2014).
Zhao, M. et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442, 457–460 (2006).
Cohen, D. J., Nelson, W. J. & Maharbiz, M. M. Galvanotactic control of collective cell migration in epithelial monolayers. Nat. Mater. 13, 409–417 (2014).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Jiang, Y. et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022).
Power, G., Moore, Z. & O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: a systematic review. J. Wound Care 26, 381–397 (2017).
Kelly-O’Flynn, S., Mohamud, L. & Copson, D. Medical adhesive-related skin injury. Br. J. Nurs. 29, S20–S26 (2020).
Fumarola, S. et al. Overlooked and underestimated: medical adhesive-related skin injuries. J. Wound Care 29, S1–S24 (2020).
Schild, H. G. Poly(N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 17, 163–249 (1992).
Cao, S., Tong, X., Dai, K. & Xu, Q. A super-stretchable and tough functionalized boron nitride/PEDOT:PSS/poly(Nisopropylacrylamide)hydrogel with self-healing, adhesion, conductive and photothermal activity. J. Mater. Chem. A 7, 8204–8209 (2019).
Fundueanu, G., Constantin, M. & Ascenzi, P. Poly(N-isopropylacrylamide-co-acrylamide) cross-linked thermoresponsive microspheres obtained from preformed polymers: influence of the physico-chemical characteristics of drugs on their release profiles. Acta Biomater. 5, 363–373 (2009).
Zhang, Q., Weber, C., Schubert, U. S. & Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: from fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 4, 109–116 (2017).
Negut, I., Grumezescu, V. & Grumezescu, A. M. Treatment strategies for infected wounds. Molecules 23, 2392 (2018).
Chen, H. et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci. Adv. 6, eaba4311 (2020).
Wu, J. & Yan, L. J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 8, 181–188 (2015).
Schutzius, G. et al. BET bromodomain inhibitors regulate keratinocyte plasticity. Nat. Chem. Biol. 17, 280–290 (2021).
Mahmoudi, S. et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 574, 553–558 (2019).
Chen, K. et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 12, 5256 (2021).
Trotsyuk, A. A. et al. Inhibiting fibroblast mechanotransduction modulates severity of idiopathic pulmonary fibrosis. Adv. Wound Care 11, 511–523 (2022).
Barrera, J. A. et al. Adipose-derived stromal cells seeded in pullulan-collagen hydrogels improve healing in murine burns. Tissue Eng. Part A 27, 844–856 (2021).
Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H. & Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585–601 (2008).
Chen, K. et al. Mechanical strain drives myeloid cell differentiation toward proinflammatory subpopulations. Adv. Wound Care 11, 466–478 (2022).
Kim, S. Y. & Nair, M. G. Macrophages in wound healing: activation and plasticity. Immunol. Cell Biol. 97, 258–267 (2019).
Wynn, T. A. & Vannella, K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462 (2016).
Duyverman, A. M., Kohno, M., Duda, D. G., Jain, R. K. & Fukumura, D. A transient parabiosis skin transplantation model in mice. Nat. Protoc. 7, 763–770 (2012).
Sîrbulescu, R. F. et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen. 25, 774–791 (2017).
Hofmann, U. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 (2012).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Wernig, G. et al. Unifying mechanism for different fibrotic diseases. Proc. Natl Acad. Sci. USA 114, 4757–4762 (2017).
Wang, J. et al. High expression of Fibronectin 1 suppresses apoptosis through the NF-κB pathway and is associated with migration in nasopharyngeal carcinoma. Am. J. Transl. Res. 9, 4502–4511 (2017).
Farr, L., Ghosh, S. & Moonah, S. Role of MIF cytokine/CD74 receptor pathway in protecting against injury and promoting repair. Front. Immunol. 11, 1273 (2020).
Carlson, B. A. et al. Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol. 10, 57 (2009).
Lin, J. D. et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4, e124574 (2019).
Huang, Z. H., Reardon, C. A. & Mazzone, T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes 55, 3394–3402 (2006).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Arnold, L. et al. CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. Nat. Commun. 6, 8972 (2015).
Wang, Y. et al. Tissue-resident macrophages promote extracellular matrix homeostasis in the mammary gland stroma of nulliparous mice. eLife 9, e57438 (2020).
Li, C., Levin, M. & Kaplan, D. L. Bioelectric modulation of macrophage polarization. Sci. Rep. 6, 21044 (2016).
Hoare, J. I., Rajnicek, A. M., McCaig, C. D., Barker, R. N. & Wilson, H. M. Electric fields are novel determinants of human macrophage functions. J. Leukoc. Biol. 99, 1141–1151 (2016).
Duscher, D. et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci. Rep. 4, 7144 (2014).
Tavares Pereira, Do. S., Lima-Ribeiro, M. H., de Pontes-Filho, N. T., Carneiro-Leão, A. M. & Correia, M. T. Development of animal model for studying deep second-degree thermal burns. J. Biomed. Biotechnol. 2012, 460841 (2012).
Fila, G. et al. Murine model imitating chronic wound infections for evaluation of antimicrobial photodynamic therapy efficacy. Front. Microbiol. 7, 1258 (2016).
Wong, V. W., Sorkin, M., Glotzbach, J. P., Longaker, M. T. & Gurtner, G. C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011, 969618 (2011).
Walmsley, G. G. et al. Murine dermal fibroblast isolation by FACS. J. Vis. Exp. 107, e53430 (2016).
Liu, Y., Keikhosravi, A., Mehta, G. S., Drifka, C. R. & Eliceiri, K. W. Methods for quantifying fibrillar collagen alignment. Methods Mol. Biol. 1627, 429–451 (2017).
Bredfeldt, J. S. et al. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J. Biomed. Opt. 19, 16007 (2014).
Kam, Y. et al. Nest expansion assay: a cancer systems biology approach to in vitro invasion measurements. BMC Res. Notes 2, 130 (2009).
Chen, K. et al. Role of boundary conditions in determining cell alignment in response to stretch. Proc. Natl Acad. Sci. USA 115, 986–991 (2018).
Wong, V. W. et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 18, 148–152 (2011).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Diaz-Papkovich, A., Anderson-Trocmé, L., Ben-Eghan, C. & Gravel, S. UMAP reveals cryptic population structure and phenotype heterogeneity in large genomic cohorts. PLoS Genet. 15, e1008432 (2019).
Mostafavi, S. et al. Variation and genetic control of gene expression in primary immunocytes across inbred mouse strains. J. Immunol. 193, 4485–4496 (2014).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Li, D. Q. et al. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul. Surf. 20, 20–32 (2021).
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Acknowledgements
This work was supported by the Stanford Clinical and Translational Science Award (CTSA) to Spectrum. The CTSA program is led by the National Center for Advancing Translational Sciences at NIH. Part of this work was performed at Stanford Nano Shared Facilities, supported by the National Science Foundation under award no. ECCS-2026822. We thank Agfa for providing PEDOT:PSS. We thank T. Carlomagno and T. Vang for administrative support. We thank Y. J. Park for tissue histology support, and D. Wu at Stanford Animal Histology Services and P. Chu at the Human Research Histology Core for help with preparation of histologic specimens. We thank S. Kananian for instrument support with VNA measurements. We also thank R. Altman for his guidance with the project.
Author information
Authors and Affiliations
Contributions
Y.J., A.A.T., S.N., G.C.G. and Z.B. designed the study. S.N. and Y.J. performed circuit design and testing. Y.J., C.-C.S., J.-C.L., D.Z. and J.T. performed material synthesis and characterizations. A.A.T., Y.J., D.H., K.C., A.M.M.-B., S.M., M.R.L., A.S., E.B., S.J., S.R.S., K.S., T.J., E.Z., C.R.N., W.G.V., D.S., J.P., M.R., D.P.P., A.C., M.C.L., C.A.B., S.H.K., K.S.F., G.G., K.L. and K.Z. performed animal and cell culture experiments and single-cell evaluations. Y.J., A.A.T., S.N., M.J., G.C.G. and Z.B. wrote the manuscript with input from all coauthors.
Corresponding authors
Ethics declarations
Competing interests
Y.J., A.A.T., S.N., G.C.G. and Z.B. have filed a provisional application of patent through Stanford University with the assigned application number 63/238,017. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Can Dincer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictctional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43 and Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Trotsyuk, A.A., Niu, S. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat Biotechnol 41, 652–662 (2023). https://doi.org/10.1038/s41587-022-01528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-022-01528-3
This article is cited by
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
A flexible bioelectronic bandage for accelerated repair of intestinal wounds
Nature Electronics (2024)
-
Accelerated intestinal wound healing via dual electrostimulation from a soft and biodegradable electronic bandage
Nature Electronics (2024)
-
Gelatin-Based Metamaterial Hydrogel Films with High Conformality for Ultra-Soft Tissue Monitoring
Nano-Micro Letters (2024)
-
Bionic artificial skin with a fully implantable wireless tactile sensory system for wound healing and restoring skin tactile function
Nature Communications (2024)