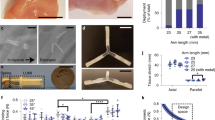
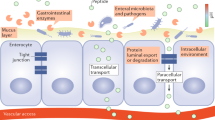
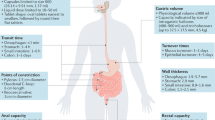
Abstract
Oral administration provides a simple and non-invasive approach for drug delivery. However, due to poor absorption and swift enzymatic degradation in the gastrointestinal tract, a wide range of molecules must be parenterally injected to attain required doses and pharmacokinetics. Here we present an orally dosed liquid auto-injector capable of delivering up to 4-mg doses of a bioavailable drug with the rapid pharmacokinetics of an injection, reaching an absolute bioavailability of up to 80% and a maximum plasma drug concentration within 30 min after dosing. This approach improves dosing efficiencies and pharmacokinetics an order of magnitude over our previously designed injector capsules and up to two orders of magnitude over clinically available and preclinical chemical permeation enhancement technologies. We administered the capsules to swine for delivery of clinically relevant doses of four commonly injected medications, including adalimumab, a GLP-1 analog, recombinant human insulin and epinephrine. These multi-day dosing experiments and oral administration in awake animal models support the translational potential of the system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data used to generate the figures and numerical statistics in this paper can be found as attached Excel files in the Supplementary Information. Source data are provided with this paper.
References
Boye, K. S. et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur. J. Heal. Econ. 12, 219–230 (2011).
Pratley, R. E. et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375, 1447–1456 (2010).
Turner, R. C., Cull, C. A., Frighi, V. & Holman, R. R.Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281, 2005–2012 (1999).
Colombel, J. F. et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 132, 52–65 (2007).
Brayden, D. J. & Alonso, M.-J. Oral delivery of peptides: opportunities and issues for translation. Adv. Drug Deliv. Rev. 106, 193–195 (2016).
Drucker, D. J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 19, 277–289 (2019).
Rubino, A., McQuay, L. J., Gough, S. C., Kvasz, M. & Tennis, P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with type 2 diabetes: a population-based analysis in the UK. Diabet. Med. 24, 1412–1418 (2007).
Ruemmele, F. M. et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohn’s Colitis 8, 1179–1207 (2014).
Ahadian, S. et al. Micro and nanoscale technologies in oral drug delivery. Adv. Drug Deliv. Rev. 157, 37–62 (2020).
Anselmo, A. C., Gokarn, Y. & Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 18, 19–40 (2018).
Buckley, S. T. et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 10, eaar7047 (2018).
Pratley, R. et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 394, 39–50 (2019).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Halberg, I. B. et al. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 7, 179–188 (2019).
Abramson, A., Halperin, F., Kim, J. & Traverso, G. Quantifying the value of orally delivered biologic therapies: a cost-effectiveness analysis of oral semaglutide. J. Pharm. Sci. 108, 3138–3145 (2019).
Mahmood, A. & Bernkop-Schnürch, A. SEDDS: a game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug Deliv. Rev. 142, 91–101 (2019).
Phan, T. N. Q., Shahzadi, I. & Bernkop-Schnürch, A. Hydrophobic ion-pairs and lipid-based nanocarrier systems: the perfect match for delivery of BCS class 3 drugs. J. Controlled Release 304, 146–155 (2019).
Fox, C. B. et al. Fabrication of sealed nanostraw microdevices for oral drug delivery. ACS Nano 10, 5873–5881 (2016).
Banerjee, A., Wong, J., Gogoi, R., Brown, T. & Mitragotri, S. Intestinal micropatches for oral insulin delivery. J. Drug Target. 25, 608–615 (2017).
Melmed, S. et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J. Clin. Endocrinol. Metab. 100, 1699–1708 (2015).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Abramson, A. et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 25, 1512–1518 (2019).
Hashim, M. et al. Jejunal wall delivery of insulin via an ingestible capsule in anesthetized swine—a pharmacokinetic and pharmacodynamic study. Pharmacol. Res. Perspect. 7, e00522 (2019).
Dhalla, A. K. et al. A robotic pill for oral delivery of biotherapeutics: safety, tolerability, and performance in healthy subjects. Drug Deliv. Transl. Res. https://doi.org/10.1007/s13346-021-00938-1 (2021).
Banerjee, A. et al. Ionic liquids for oral insulin delivery. Proc. Natl Acad. Sci. USA 115, 7296–7301 (2018).
Angsantikul, P. et al. Ionic liquids and deep eutectic solvents for enhanced delivery of antibodies in the gastrointestinal tract. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202002912 (2020).
Mathiowitz, E. et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature 386, 410–414 (1997).
Lamson, N. G., Berger, A., Fein, K. C. & Whitehead, K. A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 4, 84–96 (2020).
Bolondi, L. et al. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology 89, 752–759 (1985).
Derrickson, B. H. & Tortora, G. J. Principles of Anatomy and Physiology (Wiley, 2008).
Várkonyi, P. L. & Domokos, G. Mono-monostatic bodies: the answer to Arnold’s question. Math. Intell. 28, 34–38 (2006).
Butterworth, J. R., Wright, K., Boulton, R. A., Pathmakanthan, S. & Goh, J. Management of swallowed razor blades— retrieve or wait and see? Gut 53, 475–477 (2004).
Velitchkov, N. G., Grigorov, G. I., Losanoff, J. E. & Kjossev, K. T. Ingested foreign bodies of the gastrointestinal tract: retrospective analysis of 542 cases. World J. Surg. 20, 1001–1005 (1996).
Traverso, G. et al. Microneedles for drug delivery via the gastrointestinal tract. J. Pharm. Sci. 104, 362–367 (2015).
Webb, W. A. Management of foreign bodies of the upper gastrointestinal tract: update. Gastrointest. Endosc. 41, 39–51 (1995).
Bass, D. M., Prevo, M. & Waxman, D. S. Gastrointestinal safety of an extended-release, nondeformable, oral dosage form (OROS): a retrospective study. Drug Saf. 25, 1021–1033 (2002).
Ben-Menachem, T. et al. Adverse events of upper GI endoscopy. Gastrointest. Endosc. 76, 707–718 (2012).
Ginsberg, G. G. Management of ingested foreign objects and food bolus impactions. Gastrointest. Endosc. 41, 33–38 (1995).
Coffman, C. et al. Particles comprising a therapeutic or diagnostic agent and suspensions and methods of use thereof. US Patent Application 20190374470A1 (2019).
Savjani, K. T., Gajjar, A. K. & Savjani, J. K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012, 1–10 (2012).
Acknowledgements
We thank J. Haupt, M. Jamiel, C. Cleveland, C. Anker, A. Benfeldt, C. Jensen, H. Toftelund and A. H. Uhrenfeldt for help with in vivo porcine work. We thank the MIT Koch Institute Swanson Biotechnology Center histology and high-throughput cores for technical support. We thank U. Stilz, T. Kjeldsen, L. F. Iversen, M. Bielecki and P. B. Nielsen for discussions about SOMA development. We are grateful to all members of Langer and Traverso laboratories and Novo Nordisk for their expertise around biologic drug delivery. We thank the team at GTReel Productions, specifically Giancarlo Traverso, for assistance with assembly of the supplementary videos. Work was funded, in part, by a Novo Nordisk grant (R.L. and G.T.), National Institutes of Health grant no. EB-000244 (R.L. and G.T.), National Science Foundation GRFP fellowship (A.A.), Karl Van Tassel (1925) Career Development Professorship, Department of Mechanical Engineering, Massachusetts Institute of Technology and Division of Gastroenterology, Brigham and Women’s Hospital (G.T.) and the Viking Olof Björk scholarship trust (N.R.).
Author information
Authors and Affiliations
Contributions
A.A., M.R.F., R.L. and G.T. developed the concept. A.A., M.R.F., M.O.J., J. Windum, B.M. and B.J. designed the device. M.W.H.L. and M.P. performed injection characterization studies. A.A., J. Wainer and X.L. performed high-speed video experiments. A.A., A.V., E.M.S., J.C., S.T., K.I. and A.H. performed in vivo experiments. J.J.W. and A.B. developed liquid formulations and dissolving pellets. F.H. performed activity and stability assays on liquid formulations. J.F., S.B.G. and A.V. performed analysis on in vivo data. R.K.K. performed histology. U.R., S.T.B., P.H., N.R., R.L. and G.T. had oversight and leadership responsibility for the research activity planning and execution. A.A., M.R.F., A.V., R.L. and G.T. wrote the manuscript. All authors discussed results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.R.F., M.P., A.V., B.M., F.H., J.J.W., J.F., R.K.K., S.B.G., E.M.S., S.T.B., P.H., M.O.J., J. Windum, A.B., E.S. and U.R. are employees of Novo Nordisk. M.W.H.L. and B.J. are hired as consultants for Novo Nordisk. A.A., M.R.F., A.V., M.P., J.J.W., M.W.H.L., B.J., J. Windum, J. Wainer, X.L., N.R., U.R., G.T. and R.L. are co-inventors on patent applications describing oral biologic drug delivery. A.A., R.L. and G.T. report receiving consulting fees from Novo Nordisk. A.A. reports receiving consulting fees from Eli Lilly. Complete details of all relationships for profit and not for profit for G.T. can found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0. For a list of entities with which R.L. is involved, compensated or uncompensated, see https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0.
Additional information
Peer review information Nature Biotechnology thanks Maria Jose Alonso, David Brayden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Liquid Formulation Stability.
Human Insulin (HI) concentrated to 12.5 mg/mL and a semaglutide (Sema) exploratory formulation concentrated to 50 mg/mL were placed inside of either an L-SOMA or a glass vial and were subjected to a 40 °C and 75% relative humidity environment for two weeks. (a) Purity loss and (b) high molecular weight protein (HMWP) formation were then measured. (Mean ± SD, n = 3 device replicates; Unpaired t test).
Extended Data Fig. 2 L-SOMA needle injection images.
(a) High speed photography of an initial prototype L-SOMA device with a 21 G needle actuating into 0.3% agarose gel demonstrated that all of the liquid exits through the needle tip and none of the liquid exits through the bottom membrane. (Images are from one of n = 3 technical replicates). (b) No drug is delivered if the needle lacks a side channel (Images are from one of n = 3 technical replicates). Prototypes used during future ex vivo and in vivo studies employed a 32 G needle rather than a 21 G needle. (n = 3 technical replicates). (c) Hole on top of the L-SOMA device allows access to the (d) dissolving pellet, which actuates the needle injection. (e–g) Sequential MicroCT images of a non-retracting L-SOMA delivering contrast dye into ex vivo swine tissue. (Images are from one of n=8 technical replicates). Scales Bars = 5 mm.
Extended Data Fig. 3 Injection test mechanism setup and ex vivo injection force calculations.
(a) (Top) Computer aided design of the custom actuation mechanism used to insert a needle a controlled distance and inject an exact amount of fluid. (Bottom) Experimental setup of controlled injection studies. The texture analyzer pushes down on the plunger which causes the liquid to inject into the swine stomach tissue below. (b, c) The force required to inject a depot into ex vivo swine stomach tissue at a given needle insertion depth. (Mean ± SD; 4 mm: n=11; 4.5 mm: n=9; 5 mm: n=18). (d, e) The force required to inject a depot into ex vivo swine stomach tissue using a needle with a 10° backgrind and a backgrind of >50°. (Mean ± SD; 10°: n=20; >50°: n=5; Unpaired t test).
Extended Data Fig. 4 Dog ex vivo histology from L-SOMA injection.
Histology of ex vivo dog stomach after an L-SOMA insulin injection using a (a) hematoxylin and eosin stain, (b) an immunohistochemistry stain against insulin, (c) or an immunohistochemistry stain against smooth muscle actin. These are example images from one of 3 replicates. Scale bar = 1 mm.
Extended Data Fig. 5 L-SOMA needle retraction mechanism design.
The device first actuates after the hub pellet (red) dissolves and allows the latching mechanism at the top of the pill to release. The retraction spring, located axially around the needle shaft, compresses after the first stage of actuation due to the inherent movement of the needle during injection. The dissolution of the second pellet, which is exposed only after the first pellet dissolves, frees the spring to expand and draw the needle back into the capsule.
Extended Data Fig. 6 Blood glucose change in swine after 0.24 mg epinephrine injection.
Colored lines represent individual swine profiles and black lines represent the mean values.
Extended Data Fig. 7 In vivo swine histology after L-SOMA epinephrine injection.
Hematoxylin and eosin stain histology of swine stomach hours after L-SOMA injection. L-SOMA devices were injected in the fundus or body of the stomachs. An increase in cellularity is seen between the control and the experimental tissues, but this variability is expected in tissue locations with rapidly dividing cells. Moreover, the regular injection of epinephrine in the stomach during endoscopy supports the safety of intermittent administration of this drug in this location. The control histology is of swine stomach tissue not dosed with any device. These are example images from one of three animal replicates.
Extended Data Fig. 8 Pharmacokinetics of gavage dosed epinephrine and adalimumab.
Dissolved (a) adalimumab (4 mg) or (b) epinephrine (0.24 mg) were dosed through an endoscope into the lumen of swine stomachs (n=3 animal replicates).
Extended Data Fig. 9 Histology taken from swine dosed multiple times with L-SOMAs over three days.
(a–c) Three samples were collected according to standard histopathological procedures representing the (a) cardia, the (b) fundic, and the (c) pyloric region. No treatment related findings were observed in these standard samples in any of the three swine. (d) Histology from a minimal focal lesion from the fundic region of one of the three swine. The image shows an acute minimal erosion with sloughing of epithelial cells and peripheral acute hemorrhage. This injury is most likely a mechanical trauma caused by the endoscope when dosing the animal and is not a direct effect of the intended L-SOMA dosing. (e) Zoomed in image of “d”. (a-c: Scale Bar = 500 µm; d: Scale Bar = 1 mm.; e: Scale Bar = 250 µm).
Extended Data Fig. 10 SOMA actuation in dog stomach after oral dosing in an awake dog.
A radiograph of the SOMA device (a) before and (b) after actuating in the gastric cavity. Note the extension of the spring in the right panel. Scale Bar = 5 mm.
Supplementary information
Supplementary Video 1
Ex vivo actuation of a retractable L-SOMA capsule captured using MicroCT
Supplementary Video 2
In vivo delivery of radio-opaque contrast dye to the stomach wall using an L-SOMA capsule
Supplementary Data 1
Calculations of bioavailability in swine for L-SOMA-dosed insulin and GLP-1
Supplementary Data 2
Actuation times of L-SOMA after delivery in swine
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Rights and permissions
About this article
Cite this article
Abramson, A., Frederiksen, M.R., Vegge, A. et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat Biotechnol 40, 103–109 (2022). https://doi.org/10.1038/s41587-021-01024-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-021-01024-0
This article is cited by
-
End-to-end design of ingestible electronics
Nature Electronics (2024)
-
Customer-centric product presentations for monoclonal antibodies
AAPS Open (2023)
-
A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy
Nature Biomedical Engineering (2023)
-
Towards multifunctional robotic pills
Nature Biomedical Engineering (2023)
-
Bioinspired oral delivery devices
Nature Reviews Bioengineering (2023)