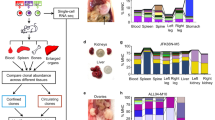
Abstract
Genetic and non-genetic heterogeneity within cancer cell populations represent major challenges to anticancer therapies. We currently lack robust methods to determine how preexisting and adaptive features affect cellular responses to therapies. Here, by conducting clonal fitness mapping and transcriptional characterization using expressed barcodes and single-cell RNA sequencing (scRNA-seq), we have developed tracking differential clonal response by scRNA-seq (TraCe-seq). TraCe-seq is a method that captures at clonal resolution the origin, fate and differential early adaptive transcriptional programs of cells in a complex population in response to distinct treatments. We used TraCe-seq to benchmark how next-generation dual epidermal growth factor receptor (EGFR) inhibitor–degraders compare to standard EGFR kinase inhibitors in EGFR-mutant lung cancer cells. We identified a loss of antigrowth activity associated with targeted degradation of EGFR protein and an essential role of the endoplasmic reticulum (ER) protein processing pathway in anti-EGFR therapeutic efficacy. Our results suggest that targeted degradation is not always superior to enzymatic inhibition and establish TraCe-seq as an approach to study how preexisting transcriptional programs affect treatment responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are deposited to the European Genome–Phenome Archive (EGAS00001005405) and ArrayExpress (E-MTAB-10698). Source data are provided with this paper.
Code availability
No custom algorithms were used in the study. Full code will be made available upon request.
References
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Lito, P., Rosen, N. & Solit, D. B. Tumor adaptation and resistance to RAF inhibitors. Nat. Med. 19, 1401–1409 (2013).
Choi, Y. L. et al. EML4–ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med. 363, 1734–1739 (2010).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR–ABL gene mutation or amplification. Science 293, 876–880 (2001).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2, e73 (2005).
Yauch, R. L. et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin. Cancer Res. 11, 8686–8698 (2005).
Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 15, 271–286 (2018).
Kitai, H. et al. Epithelial-to-mesenchymal transition defines feedback activation of receptor tyrosine kinase signaling induced by MEK inhibition in KRAS-mutant lung cancer. Cancer Discov. 6, 754–769 (2016).
Uramoto, H. et al. Epithelial-mesenchymal transition in EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res. 30, 2513–2517 (2010).
Burslem, G. M. & Crews, C. M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell 181, 102–114 (2020).
Verma, R., Mohl, D. & Deshaies, R. J. Harnessing the power of proteolysis for targeted protein inactivation. Mol. Cell 77, 446–460 (2020).
Olson, C. M. et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat. Chem. Biol. 14, 163–170 (2018).
You, I. et al. Discovery of an AKT degrader with prolonged inhibition of downstream signaling. Cell Chem. Biol. 27, 66–73 (2020).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Weinreb, C., Rodriguez-Fraticelli, A., Camargo, F. D. & Klein, A. M. Lineage tracing on transcriptional landscapes links state to fate during differentiation. Science 367, eaaw3381 (2020).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Sankar, K., Gadgeel, S. M. & Qin, A. Molecular therapeutic targets in non-small cell lung cancer. Expert Rev. Anticancer Ther. 20, 647–661 (2020).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Leonetti, A. et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 121, 725–737 (2019).
Buckley, D. et al. Targeting the von Hippel–Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J. Am. Chem. Soc. 134, 4465–4468 (2012).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015).
Shah, K. N. et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat. Med. 25, 111–118 (2019).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Byers, L. A. et al. An epithelial–mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19, 279–290 (2013).
Okura, N. et al. ONO-7475, a novel AXL inhibitor, suppresses the adaptive resistance to initial EGFR-TKI treatment in EGFR-mutated non-small cell lung cancer. Clin. Cancer Res. 26, 2244–2256 (2020).
Taniguchi, H. et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat. Commun. 10, 259 (2019).
Zhang, Z. et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 44, 852–860 (2012).
Hata, A. N. et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 22, 262–269 (2016).
Wagle, M. C. et al. A transcriptional MAPK pathway activity score (MPAS) is a clinically relevant biomarker in multiple cancer types. NPJ Precis. Oncol. 2, 7 (2018).
Jia, Y. et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 534, 129–132 (2016).
To, C. et al. Single and dual targeting of mutant EGFR with an allosteric inhibitor. Cancer Discov. 9, 926–943 (2019).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Timosenko, E. et al. Nutritional stress induced by tryptophan-degrading enzymes results in ATF4-dependent reprogramming of the amino acid transporter profile in tumor cells. Cancer Res. 76, 6193–6204 (2016).
Rabouw, H. H. et al. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc. Natl Acad. Sci. USA 116, 2097–2102 (2019).
Stockwell, S. et al. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signaling. PLoS ONE 7, e28568 (2012).
Bruch, J. et al. PERK activation mitigates tau pathology in vitro and in vivo. EMBO Mol. Med. 9, 371–384 (2017).
Yu, M. et al. A resource for cell line authentication, annotation and quality control. Nature 520, 307–311 (2015).
Galdeano, C. et al. Structure-guided design and optimization of small molecules targeting the protein–protein interaction between the von Hippel–Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) ɑ subunit with in vitro nanomolar affinities. J. Med. Chem. 57, 8657–8663 (2014).
Blaquiere, N. et al. Preparation of (4-hydroxypyrrolodin-2-yl)-hydroxamate compounds and methods of use thereof as modulators of targeted ubiquitination. WO2019084026 (2019).
Schnur, R. C. et al. Preparation of N-phenylquinazoline-4-amines as neoplasm inhibitors. WO9630347 (1996).
Blaquiere, N. et al. Hetero-bifunctional degrader compounds and their use as modulators of targeted ubiquination (VHL). WO2019183523 (2019).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Acknowledgements
We thank E. Plise, M. Beresini, H. La and F. Hsu for helping with compound characterizations.
Author information
Authors and Affiliations
Contributions
M.T.C., M.E., S.M., S.A.F. and X.Y. designed the study. Y.A.Y. and Z.M. designed and performed scRNA-seq. M.T.C., T.T.T.N. and R.P. performed the bioinformatics analysis. B.H. and X.Y. designed the TraCe-seq vector. F.S., S.A.F. and X.Y. performed cell culture experiments. S.T.S., L.G. and I.E.W. designed the EGFR degraders. S.Y. synthesized the EGFR CIDEs. The manuscript was written by M.T.C., S.A.F. and X.Y. and included contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
All authors are or were employed by Genentech Inc., South San Francisco, CA, USA, at the time of their contribution to this work.
Additional information
Peer review information Nature Biotechnology thanks Leor Weinberger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quality control metrics for TraCe-seq barcode recovery and assignment.
(a) Single-cell RNA-seq results obtained from a mixture of five cell lines labeled with different TraCe-seq barcodes respectively were visualized using UMAP. Clustering was performed based on transcriptomic differences (left) and annotated by TraCe-seq barcode assigned (right). Majority of cells of a given TraCe-seq barcode label corresponded to a specific cell line/cluster. (b) Heatmaps of top 3 marker genes from each cluster based on TraCe-seq annotation in bulk RNA-seq (left panel) and scRNA-seq (right panel). (c) Violin plots showing expression of marker genes in each cell line among the mixture. (d) The panel on the left shows FACS enrichment for the top 50% eGFP-expressing cells (n = 20,000 cells were plotted). The four panels on the right show dropout rate of TraCe-seq barcodes compared to endogenously expressed genes before and after FACS sorting in NCI-H1373 (920 unsorted cells, 3,987 sorted cells) and PC9 (965 unsorted cells, 21,043 sorted cells) cells. The red points represent the TraCe-seq barcodes, and blue points represent groups of endogenous genes with the same median raw counts shown on the x-axis. Error bars specified 2 standard deviations from the median dropout rate for the endogenous genes. (e) Box plot showing cells with mis-assigned TraCe-seq barcodes had significantly lower barcode-expression level (MIS 473 cells, COR 1,562 cells). The bottom side of the box represents the first quartile, and the top side, the third quartile. The line represents the median. p value was determined using two-tailed Student’s t-test.
Extended Data Fig. 2 Characterization of GNE-104 and GNE-069.
(a) Western blot showing dose-dependent EGFR degradation induced by GNE-104 in HCC827 cells. This extended dilution series related to panel (b) was only conducted once. (b) Western blot showing effect on EGFR and pEGFR by GNE-104 versus GNE-069 in HCC827 cells. Results are representative of 2 independent experiments. (c) Western blot showing excess free VHL ligand inhibits GNE-104 induced EGFR protein degradation in HCC827 cells, while no effects were observed for GNE-069. This experiment was repeated 2 times with similar results. (d) Characterization of the biochemical potency (towards common EGFR mutant variants) and selectivity (against a panel of 218 kinases) of GNE-104 and GNE-069 in in vitro kinase inhibition assays.
Extended Data Fig. 3 Characterization of the response of PC9 cells to erlotinib, GNE-069, and GNE-104.
(a) Heatmap and dendrogram showing survival patterns of 500 different PC9 clones subject to erlotinib (2 µM) or GNE-104 (1 µM) treatment for two months. Clonal abundance was determined by NGS barcode analyses from genomic DNA. Barcode enrichment were highly reproducible within individual treatment and differed between erlotinib and GNE-104 treatments. (b–d) Comparison of the anti-growth effects of erlotinib, GNE-069, and GNE-104 in PC9 cells by (b) clonogenic assay, scale bar, 1 mm, (c) cell counting in triplicates (200 K cells per well in 6-well plates), and (d) Incucyte imaging, scale bar, 200 µm. All three compounds were applied at 1 µM. The experiments were repeated 3 times with similar results. Erlotinib and GNE-069 had comparable activity by all three measures, whereas GNE-104 was less efficacious.
Extended Data Fig. 4 TraCe-seq barcode enrichment and depletion analyses.
(a) Deep NGS sequencing of TraCe-seq clonal abundance derived from genomic DNA (x-axis) correlates with scRNA-seq derived TraCe-seq clonal abundance (y-axis). Cutoff (red) shown of minimum TraCe-seq barcode for downstream clonal depletion analyses shown in panel (d). (b) Pairwise comparisons of TraCe-seq clonal abundance under different treatment conditions, x and y-axes are abundance of a given TraCe-seq barcoded clone under indicated conditions labeled at the top and to the right of the plots respectively. Pearson correlation coefficient of the barcode abundance distributions are shown. (c) Distribution of log2 fold change of TraCe-seq barcode in each treatment condition compared to baseline. (d) Heatmap showing relative abundance of depleted TraCe-seq barcodes upon erlotinib, GNE-069, or GNE-104 treatments.
Extended Data Fig. 5 Clonogenic assay confirming differential activity of EGFR kinase inhibitors versus degrader GNE-104.
(a) Clonogenic assay showing differential anti-growth effects of GNE-104 compared to erlotinib or GNE-069 across four different EGFR-mutant lung cancer cell lines. Scale bar, 1 mm. (b) Quantification of relative viability of the same four EGFR-mutant lung cancer cell lines shown in panel (a) under degrader GNE-104 or non-degrader control GNE-069 treatment relative to erlotinib over 14 days using CellTiter-Glo luminescent cell viability assay. Experiments in (a, b) were run in parallel. Results are representative of 2–4 independent experiments (depending upon the cell line). (c) Clonogenic assay showing that high concentration of free VHL ligand (10 µM) did not affect cellular response to erlotinib or GNE-069 in PC9 cells. The VHL inactive enantiomer was included as a further control. Scale bar, 2 mm. Similar results were observed using two different active VHL ligands.
Extended Data Fig. 6 Abundance of treatment resistant versus sensitive TraCe-seq barcodes among inferred trajectories.
(a) Density plot showing distribution of cells with kinase inhibitor resistant versus kinase inhibitor sensitive TraCe-seq barcodes in the UMAP space subject to erlotinib/GNE-069 treatment. Each grey dot represents an individual cell. (b) Comparison of resistant versus sensitive barcode category distributions at the end UMAP cluster of the four inferred trajectories. Path a, b, and c were each dominated by at least one resistant clone category (inferred to represent adaptation/resistance), while Path d had the highest relative abundance of drug sensitive clones thus were inferred to represent response. (c) Density plot showing distribution of cells with degrader resistant barcodes subject to GNE-104 treatment versus kinase inhibitor erlotinib/GNE-069 treatments. Each grey dot represents an individual cell.
Extended Data Fig. 7 Additional characterization of cells treated with siEGFR and kinase inhibitors or osimertinib plus allosteric EGFR degrader.
(a) Schematic showing the siEGFR experimental setup in PC9 and HCC4006 cells. (b) Western blot analysis of total EGFR and pEGFR subject to the indicated treatment for two days. The experiment was repeated 2 times with similar results. (c) Representative qRT-PCR analysis of key MAPK pathway transcriptional targets in PC9 cells treated under indicated conditions for three days. (d) Representative qRT-PCR analysis of key transcription targets of MAPK pathway in HCC4006 cells under indicated conditions for five days. (e) Clonogenic assay showing siEGFR promoted survival of HCC4006 cells under EGFR kinase inhibitors erlotinib and osimertinib treatments. Scale bar, 1 mm. The experiment was repeated 4 times with similar results. (f) Representative qRT-PCR analysis of key transcriptional targets of the MAPK pathway in NCI-H1975 cells under indicated conditions on day 3. N.D., not detected. All error bars in the bar graphs (c, d, and f) represent s.d., and the centers of the error bars represent the mean of n = 3 biologically dependent technical replicates. The experiments in panels c, d, and f were repeated 2 times (biologically independent) with similar results.
Extended Data Fig. 8 Characterization of GNE-640 and GNE-641.
(a) Chemical structures of allosteric EGFR ligand EAI-045, GNE-641 (active degrader), and GNE-640 (inactive degrader). (b) Western blot showing that addition of the free VHL ligand can rescue the EGFR degradation upon GNE-641 treatment in NCI-H1975 cells. GNE-640 was included as a further control. Similar results were observed with a related active/inactive EGFR allosteric degrader pair. (c) Clonogenic assay showing very modest single agent activity of EAI-045, GNE-640, or GNE-641 in NCI-H1975 cells compared to osimertinib. Scale bar, 1 mm. The experiment was repeated 2 times with similar results. (d) Quantifications showing relative viability of NCI-H1975 cells co-treated with 0.1 µM osimertinib and 1 µM allosteric degrader GNE-641 (or non-degrader GNE-640) compared to treated with osimertinib alone over 14 days using CellTiter-Glo luminescent cell viability assay. Viability is normalized to osimertinib alone. Results are representative of 2 independent experiments. (e) Clonogenic assay showing allosteric degrader GNE-641 but not the non-degrader control GNE-640 promoted survival of NCI-H3255 cells under osimertinib treatment. Scale bar, 4 mm. The experiment was repeated 2 times with similar results.
Extended Data Fig. 9 Pharmacological modulation of ER stress alters response to EGFR kinase inhibitors and degrader.
(a) Schematic showing pharmacological modulators of ER stress pathway. (b) Representative qRT-PCR analysis of key pro-death genes downstream of ER stress in HCC4006 cells under the indicated conditions on day 5. (c) Representative qRT-PCR quantification of pro-death integrated stress response genes downstream of ER stress and key transcriptional targets of the MAPK pathway in NCI-H1975 cells under indicated treatment conditions on day 3. N.D., not detected. (d) Clonogenic assay showing attenuation of osimertinib acitivity by ISRIB in NCI-H1975 cells. Scale bar, 1 mm. The experiment was repeated 3 times with similar results. (e) Clonogenic assay showing very low non-toxic doses of ER stress inducers effectively enhanced activity of GNE-104. Scale bar, 1 mm. The experiment was repeated 4 times with similar results. (f) Representative qRT-PCR quantifications of key pro-death genes downstream of ER stress in PC9 cells treated under the indicated conditions on day 3. Error bars in the bar graphs (b, c, and f) represent s.d., and the centers of the error bars represent the mean of n = 3 biologically dependent technical replicates. The experiments in panel b, c were repeated 2 times (biologically independent) and in panel f was repeated 3 times (biologically independent) with similar results.
Extended Data Fig. 10 PERK activator CCT020312 potentiates activity of FDA-approved EGFR kinase inhibitors.
(a–c) Representative qRT-PCR quantification of pro-death genes downstream of ER stress and MAPK target genes in PC9 cells (a, b) and NCI-H1975 cells (c) under the indicated conditions on day 3. Error bars represent s.d., and the centers of the error bars represent the mean of n = 3 biologically dependent technical replicates. These experiments were repeated 2 times (biologically independent) with similar results. (d) Quantification showing EGFRi+CCT020312 combinations were more effective in inducing cell death compared to the single agents using Caspase-Glo3/7 assay system. Error bars represent s.d., and the centers of the error bars represent the mean (n = 3 biologically independent samples). The experiment was repeated 2 times with similar results.
Supplementary information
Source data
Source Data Fig. 3
Uncropped western blots.
Source Data Extended Data Fig. 2
Uncropped western blots.
Source Data Extended Data Fig. 7
Uncropped western blots.
Source Data Extended Data Fig. 8
Uncropped western blots.
Rights and permissions
About this article
Cite this article
Chang, M.T., Shanahan, F., Nguyen, T.T.T. et al. Identifying transcriptional programs underlying cancer drug response with TraCe-seq. Nat Biotechnol 40, 86–93 (2022). https://doi.org/10.1038/s41587-021-01005-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-021-01005-3
This article is cited by
-
Pervasive transcriptome interactions of protein-targeted drugs
Nature Chemistry (2023)
-
An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance
Nature Cancer (2023)