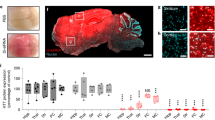
Abstract
Achieving regulation of endogenous gene expression in the central nervous system (CNS) with antisense oligonucleotides (ASOs) administered systemically would facilitate the development of ASO-based therapies for neurological diseases. We demonstrate that DNA/RNA heteroduplex oligonucleotides (HDOs) conjugated to cholesterol or α-tocopherol at the 5′ end of the RNA strand reach the CNS after subcutaneous or intravenous administration in mice and rats. The HDOs distribute throughout the brain, spinal cord and peripheral tissues and suppress the expression of four target genes by up to 90% in the CNS, whereas single-stranded ASOs conjugated to cholesterol have limited activity. Gene knockdown was observed in major CNS cell types and was greatest in neurons and microglial cells. Side effects, such as thrombocytopenia and focal brain necrosis, were limited by using subcutaneous delivery or by dividing intravenous injections. By crossing the blood–brain barrier more effectively, cholesterol-conjugated HDOs may overcome the limited efficacy of ASOs targeting the CNS without requiring intrathecal administration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its extended data and supplementary information files. Source data are provided with this paper.
References
Kole, R., Krainer, A. R. & Altman, S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 11, 125–140 (2012).
Lundin, K. E., Gissberg, O. & Smith, C. I. Oligonucleotide therapies: the past and the present. Hum. Gene Ther. 26, 475–485 (2015).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378, 625–635 (2018).
Lunn, M. R. & Wang, C. H. Spinal muscular atrophy. Lancet 371, 2120–2133 (2008).
Schwentker, E. P. & Gibson, D. A. The orthopaedic aspects of spinal muscular atrophy. J. Bone Joint Surg. Am. 58, 32–38 (1976).
Mercuri, E., Bertini, E. & Iannaccone, S. T. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 11, 443–452 (2012).
Fujak, A. et al. Natural course of scoliosis in proximal spinal muscular atrophy type II and IIIa: descriptive clinical study with retrospective data collection of 126 patients. BMC Musculoskelet. Disord. 14, 283 (2013).
Johnson, K.S. & Sexton, D.J. Lumbar puncture: technique, indications, contraindications, and complications in adults. UptoDate https://www.uptodate.com/contents/lumbar-puncture-technique-indications-contraindications-and-complications-in-adults (2018).
Pardridge, W. M. CNS drug design based on principles of blood–brain barrier transport. J. Neurochem. 70, 1781–1792 (1998).
Schoch, K. M. & Miller, T. M. Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases. Neuron 94, 1056–1070 (2017).
Dong, X. Current strategies for brain drug delivery. Theranostics 8, 1481–1493 (2018).
Nafee, N. & Gouda, N. Nucleic acids-based nanotherapeutics crossing the blood brain barrier. Curr. Gene Ther. 17, 154–169 (2017).
Zeniya, S. et al. Angubindin-1 opens the blood–brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J. Control. Release 283, 126–134 (2018).
Nishina, K. et al. DNA/RNA heteroduplex oligonucleotide for highly efficient gene silencing. Nat. Commun. 6, 7969 (2015).
Obika, S. et al. Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine: novel bicyclic nucleosides having a fixed C3, -endo sugar puckering. Tetrahedron Lett. 38, 8735–8738 (1997).
Obika, S. et al. Stability and structural features of the duplexes containing nucleoside analogues with a fixed N-type conformation, 2′-O,4′-C-methyleneribonucleosides. Tetrahedron Lett. 39, 5401–5404 (1998).
Singh, S.K., Koshkin, A.A., Wengel, J. & Nielsen, P. LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition. Chem. Commun. 4, 455–456 (1998).
Wolfrum, C. et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 25, 1149–1157 (2007).
Hung, G. et al. Characterization of target mRNA reduction through in situ RNA hybridization in multiple organ systems following systemic antisense treatment in animals. Nucleic Acid Ther. 23, 369–378 (2013).
Yu, R. Z. et al. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab. Dispos. 35, 460–468 (2007).
Geary, R. S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin. Drug Metab. Toxicol. 5, 381–391 (2009).
Nishina, T. et al. Chimeric antisense oligonucleotide conjugated to α-tocopherol. Mol. Ther. Nucleic Acids 4, e220 (2015).
Wada, S. et al. Evaluation of the effects of chemically different linkers on hepatic accumulations, cell tropism and gene silencing ability of cholesterol-conjugated antisense oligonucleotides. J. Control. Release 226, 57–65 (2016).
Seth, P. P. et al. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs. Nucleic Acids Symp. Ser. 52, 553–554 (2008).
Jauvin, D. et al. Targeting DMPK with antisense oligonucleotide improves muscle strength in myotonic dystrophy type 1 mice. Mol. Ther. Nucleic Acids 7, 465–474 (2017).
Pandey, S. K. et al. Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1. J. Pharmacol. Exp. Ther. 355, 329–340 (2015).
Bugiardini, E. & Meola, G. Consensus on cerebral involvement in myotonic dystrophy: workshop report: May 24-27, 2013, Ferrere (AT), Italy. Neuromuscul. Disord. 24, 445–452 (2014).
Hagemann, T. L. et al. Antisense suppression of glial fibrillary acidic protein as a treatment for Alexander disease. Ann. Neurol. 83, 27–39 (2018).
McCampbell, A. et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J. Clin. Invest. 128, 3558–3567 (2018).
Kordasiewicz, H. B. et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron 74, 1031–1044 (2012).
Southwell, A. L. et al. In vivo evaluation of candidate allele-specific mutant huntingtin gene silencing antisense oligonucleotides. Mol. Ther. 22, 2093–2106 (2014).
Godinho, B. et al. Transvascular delivery of hydrophobically modified siRNAs: gene silencing in the rat brain upon disruption of the blood–brain barrier. Mol. Ther. 26, 2580–2591 (2018).
Tyler, B. M. et al. Peptide nucleic acids targeted to the neurotensin receptor and administered i.p. cross the blood–brain barrier and specifically reduce gene expression. Proc. Natl Acad. Sci. USA 96, 7053–7058 (1999).
Hamzavi, R., Dolle, F., Tavitian, B., Dahl, O. & Nielsen, P. E. Modulation of the pharmacokinetic properties of PNA: preparation of galactosyl, mannosyl, fucosyl, N-acetylgalactosaminyl, and N-acetylglucosaminyl derivatives of aminoethylglycine peptide nucleic acid monomers and their incorporation into PNA oligomers. Bioconjug. Chem. 14, 941–954 (2003).
Habeck, M. PNAs a match for the BBB? Drug Discov. Today 8, 377–378 (2003).
Banks, W. A. et al. Delivery across the blood–brain barrier of antisense directed against amyloid β: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J. Pharmacol. Exp. Ther. 297, 1113–1121 (2001).
Zhang, Z. et al. Oligonucleotide-induced alternative splicing of serotonin 2C receptor reduces food intake. EMBO Mol. Med. 8, 878–894 (2016).
Hammond, S. M. et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc. Natl Acad. Sci. USA 113, 10962–10967 (2016).
Shabanpoor, F. et al. Identification of a peptide for systemic brain delivery of a morpholino oligonucleotide in mouse models of spinal muscular atrophy. Nucleic Acid Ther. 27, 130–143 (2017).
Relizani, K. et al. Efficacy and safety profile of tricyclo-DNA antisense oligonucleotides in Duchenne muscular dystrophy mouse model. Mol. Ther. Nucleic Acids 8, 144–157 (2017).
Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004).
Biscans, A. et al. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 47, 1082–1096 (2019).
Ostergaard, M. E. et al. Conjugation of hydrophobic moieties enhances potency of antisense oligonucleotides in the muscle of rodents and non-human primates. Nucleic Acids Res. 47, 6045–6058 (2019).
Swayze, E. E. et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 35, 687–700 (2007).
Rigo, F. et al. Pharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J. Pharmacol. Exp. Ther. 350, 46–55 (2014).
Ling, K. K. et al. Antisense-mediated reduction of EphA4 in the adult CNS does not improve the function of mice with amyotrophic lateral sclerosis. Neurobiol. Dis. 114, 174–183 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the \({2^{{-\Delta\Delta}{c}_{\rm{T}}}}\) method. Methods 25, 402–408 (2001).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Oude Ophuis, R. J. et al. DMPK protein isoforms are differentially expressed in myogenic and neural cell lineages. Muscle Nerve 40, 545–555 (2009).
Bruijn, L. I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Uchihara, T., Nakamura, A., Shibuya, K. & Yagishita, S. Specific detection of pathological three-repeat tau after pretreatment with potassium permanganate and oxalic acid in PSP/CBD brains. Brain Pathol. 21, 180–188 (2011).
Feldmann, M., Pathipati, P., Sheldon, R. A., Jiang, X. & Ferriero, D. M. Isolating astrocytes and neurons sequentially from postnatal murine brains with a magnetic cell separation technique. J. Biol. Methods 1, e11 (2014).
Bamford, R. A. et al. Electroporation and microinjection successfully deliver single-stranded and duplex DNA into live cells as detected by FRET measurements. PLoS ONE 9, e95097 (2014).
Acknowledgements
We thank A. Oda, Y. Kakoi and A. Kunugi (Research, Takeda Pharmaceutical Company) for conducting the in vivo studies, and S. Matsumoto and I. Chisaki (Research, Takeda Pharmaceutical Company) for quantifying the ASO and conducting PK analyses. We also thank N. Post (Ionis Pharmaceuticals) for the quantification of the ASO, M. Jackson and M. Afetian (Ionis Pharmaceuticals) for valuable technical assistance, S. Klein (Ionis Pharmaceuticals) and A. Nakamura (TMDU) for histopathological studies and A. Abe and Y. Okamoto (WDB) for their care of the laboratory animals. This research was supported by the Basic Science and Platform Technology Programs for Innovative Biological Medicine (18am0301003h0005) and Advanced Biological Medicine (20am0401006h0002) to T.Y., a Research Project on the Elucidation of Chronic Pain (19ek0610013h0003) to T.Y. and T.N. from the Japan Agency for Medical Research and Development (AMED), JST CREST (JPMJCR12L4 to T.Y.) and a JSPS KAKENHI Grant-in-Aid for Scientific Research (S) (17H06109 to T.Y. and T.N.), (A) (19H01016 to T.N. and T.Y.) and (B) (16H05221 to T.N.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Tokyo).
Author information
Authors and Affiliations
Contributions
T.N., C.A.D., F.R., P.P.S., C.F.B., Kenichi Miyata and T.Y. designed the research. T.N., C.A.D., K.Y-T., K.I., M.O., H.K., H.M., T. Ishii, S.E., K.Y., Kanjiro Miyata, Kenichi Miyata, B.P., T. Igari, S.Y., N.A., H.H., T.U., R.I.H. and T.W. performed the experiments and analyzed the results. T.N., C.A.D., P.P.S., C.F.B., F.R. and T.Y. wrote the manuscript. T.Y. and T.N. coordinated and supervised the project. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
T.Y. is collaborating with Daiichi Sankyo Co., Ltd. (Tokyo, Japan), Mitsubishi Tanabe Pharma Corporation (Osaka, Japan), NanoCarrier Co., Ltd. (Chiba, Japan), Ono Pharmaceutical Co., Ltd. (Osaka, Japan), Rena Therapeutics Inc. (Tokyo, Japan), Takeda Pharmaceutical Co., Ltd. (Tokyo, Japan) and Toray Industries, Inc. (Tokyo, Japan) and Ionis Pharmaceuticals (CA, USA). T.Y. also serves as academic adviser for Rena Therapeutics Inc. C.A.D., B.P., C.F.B., P.P.S. and F.R. are paid employees of Ionis Pharmaceuticals, Inc. Kenichi Miyata, T. Igari, S.Y., N.A. and H.H. are paid employees of Takeda Pharmaceutical Company Limited. T.Y. and T.N. are inventors on patents on BBB-HDO filed by Tokyo Medical and Dental University and have benefits from licensing of the associated intellectual property.
Additional information
Peer review information Nature Biotechnology thanks Arthur Krieg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Processing mechanism of HDO.
a, Schematic illustration of presumed processing mechanism of HDO in the cell. HDO is composed of a gapmer ASO, duplexed with a cRNA conjugated to delivery ligands. Gapmer ASOs have a central gap-region of DNA flanked by modified nucleotides that enhance affinity for cRNA, such as LNA, cEt or MOE, and whole nucleotides of ASO strand are protected from nucleases by PS modification. In the cRNA strand, the center portion hybridized to the gap portion of ASO remains unmodified for recognition of RNase H, and terminal nucleotides hybridized to both wing portions of ASO are protected from exonucleases by both PS modification and OMe. b, The effect of the cholesterol position and number in cRNA strand of Chol-HDO with LNA wings on Malat1 RNA levels. Cortex was harvested 3 days after one IV dose per week for 4 weeks of PBS or 50 mg/kg Chol-HDO (3–4/group; *P < 0.05; ** P < 0.001). c, Evaluation of Hdac2 expression in the cortex after co-administration of ASO targeting Hdac2 and Chol-HDO containing ASO with cEt wings targeting Malat1 after a single IV injection. Tissues were collected 1 week after dosing. Results show Chol-HDO does not facilitate Hdac2 ASO activity in the CNS indicating the absence of BBB damage by Chol-HDO (3/group). d, ICV administration of ASO targeting Hdac2 with cEt wings shows gene knockdown in the cerebral cortex at 30 or 100 µg doses (2/group), indicating that ASO targeting Hdac2 is effective in the CNS. Abbreviations (for all figures): ASO, antisense oligonucleotide; cEt, 2′, 4′- constrained Ethyl BNA; Chol, cholesterol; Chol-HDO, heteroduplex oligonucleotide conjugated with cholesterol; cRNA, complementary ribonucleic acid; DNA, deoxyribonucleic acid; DHA, Docosahexaenoic acid; Dmpk, dystrophia myotonica protein kinase; Gfap, glial fibrillary acidic protein; Hdac2, histone deacetylase 2; HDO, heteroduplex oligonucleotide; LNA, locked nucleic acid; Malat1, metastasis associated lung adenocarcinoma 1; MOE, 2′-O-Methoxyethyl ribonucleic acid; mRNA, messenger ribonucleic acid; OMe, 2′-O-methyl RNA ; PBS, phosphate-buffered saline; PO phosphodiester; PS phosphorothioate; RNA, ribonucleic acid; s.e.m., standard error of the mean; SOD1, superoxide dismutase 1; Tmax, time to maximal plasma concentration (Tmax); Toc, tocopherol; Toc-HDO, heteroduplex oligonucleotide conjugated with tocopherol.
Extended Data Fig. 2 The distribution and gene knockdown effect of Chol-HDO in multiple CNS regions.
a, Dose response curve showing Malat1 RNA knockdown in the spinal cord after a single IV injection of PBS or Chol-HDO with LNA or cEt wings (Dose for LNA wings, 12.5, 25, 50 or 75 mg/kg, for cEt wings 7, 20 or 50 mg/kg) (4/group). Spinal cord was harvested 3 days after dosing. Malat1 RNA levels in the cortex were normalized to Actin mRNA. Dose dependency was also observed in other brain regions with the cerebellum being the least sensitive to the HDO (data not shown). Time course of Malat1 RNA reductions in the spinal cord on 3, 7, 14, 28, 56, 168 or 252 days after a single IV injection of 50 mg/kg Chol-HDO with LNA wings (4/group; *P < 0.05; ** P < 0.001). b, The effect of 50 mg/kg Toc-HDO and Chol-HDO with LNA wings after a single IV injection on Malat1 RNA expression in various brain regions and spinal cord compared to 50 mg/kg ASO or PBS. Tissues were harvested 3 days after injection (4/group; *P < 0.05; ** P < 0.001). (c/d) In situ hybridization with a Malat1 probe in the cortex (layers I/II–VI) (c) and the anterior horn of spinal cord (d), dosing paradigm as described in Fig. 1c. Each panel (c) shows all cortical layers from the pial surface/layer I (left) to layer VI (right). The dashed lines in (d) indicate the border between white and gray matter, and the area within the dashed line is the anterior horn of spinal cord. The scale bar is 100 μm. Since Malat1 is ubiquitously expressed15, the near complete inhibition of probe signal suggests that RNA expression was reduced in both neurons and glia after Chol-HDO administration. Similar Malat1 RNA reduction was also confirmed in cerebellum, striatum, hippocampus, and brain stem (data not shown). e, Time courses of Malat1 RNA levels in the cortex and spinal cord on 7, 14 or 28 days after single SC injection of 50 mg/kg Chol-HDO with LNA wings (4–6/group; *P < 0.05; ** P < 0.001). f, Dose response curves of Malat1 gene knockdown effect comparing IV and SC injection with dosing of 50 mg/kg Chol-HDO with cEt wings once a week for four weeks (2–3/group). g, The effect of 50 mg/kg Chol-HDO with MOE gapmer ASOs on Malat1 RNA expression levels in different brain regions after a single IV injection of 50 mg/kg. Tissues were harvested 3 days after the last injection (4/group; *P < 0.05). h, The effect of Chol-HDO with cEt gapmer ASOs on Malat1 RNA in brain regions and spinal cord after IV administration of 50 mg/kg Chol-HDO once a week for four weeks. Tissues were harvested 3 days after the last injection (4/group; *P < 0.05; ** P < 0.001). i, Chol-HDO with MOE wings reduces the expression of Gfap, causative gene of Alexander disease, in various brain regions and spinal cord after IV administration of 50 mg/kg Chol-HDO once a week for four weeks. The brain and spinal cords were harvested 3 days after the last injection (4/group; *P < 0.05; ** P < 0.001). There is no clear reduction in GFAP protein in the CNS of mice treated with Chol-HDO by a Quanterix ELISA assay. While this experimental design described above was sufficient for detecting mRNA knockdown, it was too short to evaluate GFAP protein reduction as this protein is known to have a long half-life (~27 days for cortex and ~9 weeks for spinal cord). j, Chol-HDO with LNA wings reduces the expression of SOD1, the causative gene of familial amyotrophic lateral sclerosis, in various brain regions and spinal cord after SC administration of 50 mg/kg Chol-HDO once a week for four weeks (upper panel). The brain and spinal cords were harvested 7 days after the last injection (4–6/group; *P < 0.05). Human SOD1 protein expression from the brain treated with Chol-HDO was also reduced as compared to that from the brain treated with PBS on immunoblotting (lower panel). Immunoblotting and densitometry analysis (3/group; *P < 0.05) showing that Chol-HDO significantly reduced the levels of SOD1 protein applied by 10 μg total protein in brain as compared to the brain treated with PBS (lower panel).
Extended Data Fig. 3 Pharmacokinetics of Chol-HDO in CNS and plasma.
a, Time course of ASO distribution detected by anti-PS antibody staining in the cortex at 1, 24, and 48 hours after a single IV injection of 50 mg/kg Chol-HDO with LNA wings. The scale bar is 20 μm. b, Immunohistochemistry staining of anti-PS antibody in cerebral cortex, the choroid plexus and ependymal cells that line the lateral ventricle in the mouse brain 3 days after a single IV injection of 50 mg/kg single-stranded ASO or Chol-HDO with LNA wings. The ASO signals were observed in the choroid plexus (arrow) and ependymal cells (arrowhead) from mice treated with both ASO and Chol-HDO (upper panel). In contrast, the ASO signals were detected in neuron and glia in cortex from mouse treated with Chol-HDO, but not with ASO (lower panel). The scale bar is 50 μm. Lower panel show the higher magnification image of inset in upper panel. c, Ratiometric FRET imaging to localize separated cRNA and intact Chol-HDO with a Cy5-labeled ASO and a Cy3-labeled cRNA sense strand in layer I and II of the somatosensory cortex (upper panels) and cerebellum (lower panels). When the duplex is intact, the Cy3 transfers energy to Cy5 resulting in the gain of a unique FRET signal (ex540/em680). Far left heat map shows relative distribution of separated cRNA (red) and intact Chol-HDO (purple/blue/green). The majority of Chol-HDO localized at the pial basement membrane is intact and local separation is observed inside of neural cells (inset shows the neuron with close-colocalization of intact duplex and separated cRNA indicating separation of the duplex), vascular endothelial cells and ganglionic (Purkinje cell) layer of the cerebellum. The scale bar is 50 μm in the upper panel or 100 μm in the lower panel. d, Time course of ASO concentration in the plasma and cortex after a single IV injection of 50 mg/kg Chol-HDO containing ASO with LNA wings (3–4/group) on 1, 3, 7, 14, 21, 28, 42 or 56 days. As for plasma, the ASO concentration was also measured at 1, 3, 7, 14, 21, or 28 days after a single IV injection of 50 mg/kg ASO with LNA wings alone (3–4/groups). e, Comparison of Malat1 RNA levels after IV injection of 4 total dosing of 6.25 mg/kg (twice a week) and a single doing of 25 mg/kg Chol-HDO with LNA wings in different brain regions. (4/group; *P < 0.05; ** P < 0.001). f, Correlation of Malat1 RNA levels and ASO concentration in kidney after 1, 2, or 4 total IV dosing (1 dose/week) of 50 mg/kg of ASO or Chol-HDO with LNA wings (2–4/group).
Extended Data Fig. 4 Comparison of dose response between ASO and Chol-HDO by ICV injection.
a, Comparison of dose response curves generated for ASO or Chol-HDO with cEt wings targeting Malat1 by ICV bolus injection in the cortex and spinal cord (4/group). Tissues were collected 2 weeks after dosing.
Extended Data Fig. 5 Adverse effects of Chol-HDO and their mitigation by divided administrations or SC administration.
a, Platelet number and Malat1 RNA level after a single IV injection of 50 mg/kg Chol-HDO with LNA wings compared to two divided dosing of 25 mg/kg Chol-HDO with LNA wings in a week (3–12/group; *P < 0.05; ** P < 0.001). b, Platelet number and Malat1 RNA knockdown after a single IV injection or SC injection of 50 mg/kg Chol-HDO with LNA wings (10–12/group; *P < 0.05; ** P < 0.001). In all cases platelet counts and Malat1 knockdown were evaluated in the cortex at 3 days after IV injection and 7 days after SC injection. Divided or SC administration rescued thrombocytopenia observed by IV single administration of Chol-HDO. c, Histology with hematoxylin and eosin staining of the brain after once a week injection of 50 mg/kg Chol-HDO with LNA wings for four weeks. SC administration rescued focal necrosis (arrowhead) observed by IV administration of Chol-HDO (4/group). The scale bar is 300 μm. Lower panels show higher magnification images of the inset in upper panels. The arrowhead indicates necrotic neurons in the brain from intravenously repeated injected mouse. The scale bar is 50 μm.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–5
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed Western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed Western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Nagata, T., Dwyer, C.A., Yoshida-Tanaka, K. et al. Cholesterol-functionalized DNA/RNA heteroduplexes cross the blood–brain barrier and knock down genes in the rodent CNS. Nat Biotechnol 39, 1529–1536 (2021). https://doi.org/10.1038/s41587-021-00972-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-021-00972-x
This article is cited by
-
Customizing delivery nano-vehicles for precise brain tumor therapy
Journal of Nanobiotechnology (2023)
-
Amyotrophic lateral sclerosis: translating genetic discoveries into therapies
Nature Reviews Genetics (2023)
-
Genetics of amyotrophic lateral sclerosis: seeking therapeutic targets in the era of gene therapy
Journal of Human Genetics (2023)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
-
Serine/threonine kinase TBK1 promotes cholangiocarcinoma progression via direct regulation of β-catenin
Oncogene (2023)