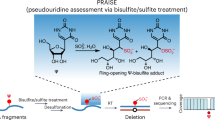
Abstract
Nanopore RNA sequencing shows promise as a method for discriminating and identifying different RNA modifications in native RNA. Expanding on the ability of nanopore sequencing to detect N6-methyladenosine, we show that other modifications, in particular pseudouridine (Ψ) and 2′-O-methylation (Nm), also result in characteristic base-calling ‘error’ signatures in the nanopore data. Focusing on Ψ modification sites, we detected known and uncovered previously unreported Ψ sites in mRNAs, non-coding RNAs and rRNAs, including a Pus4-dependent Ψ modification in yeast mitochondrial rRNA. To explore the dynamics of pseudouridylation, we treated yeast cells with oxidative, cold and heat stresses and detected heat-sensitive Ψ-modified sites in small nuclear RNAs, small nucleolar RNAs and mRNAs. Finally, we developed a software, nanoRMS, that estimates per-site modification stoichiometries by identifying single-molecule reads with altered current intensity and trace profiles. This work demonstrates that Nm and Ψ RNA modifications can be detected in cellular RNAs and that their modification stoichiometry can be quantified by nanopore sequencing of native RNA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
For in vitro transcribed datasets, FAST5 files used in this work were already publicly available (UNM and m6A: PRJNA511582) or are publicly available in the Sequence Read Archive (SRA) (m5C: PRJNA563591; hm5C: PRJNA548268; Ψ: PRJNA549001; UNM-S: PRJNA575545; Ψ with varying stoichiometries: PRJNA695584). Base-called and demultiplexed FASTQ from all yeast RNA direct RNA sequencing data runs are publicly available in the Gene Expression Omnibus (GEO) under accession number GSE148603, including processed EpiNano outputs. FAST5 files for all yeast RNA direct RNA sequencing are available in the European Nucleotide Archive (ENA) under accession numbers PRJEB37798 and PRJEB41495. A detailed description of the datasets used and sequenced in this work, with their corresponding GEO and ENA/SRA IDs, can be found in Supplementary Table 14.
Code availability
All scripts and code used in this work are available in GitHub: https://github.com/novoalab/Best_Practices_dRNAseq_analysis (analysis of in vitro curlcake datasets), https://github.com/novoalab/yeast_RNA_Mod (analysis of in vivo datasets) and https://github.com/novoalab/nanoRMS (prediction of RNA modifications and estimation of RNA modification stoichiometries).
References
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Carlile, T. M. et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515, 143–146 (2014).
Schwartz, S. et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159, 148–162 (2014).
Lovejoy, A. F., Riordan, D. P. & Brown, P. O. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS ONE 9, e110799 (2014).
Li, X. et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat. Chem. Biol. 11, 592–597 (2015).
Hussain, S., Aleksic, J., Blanco, S., Dietmann, S. & Frye, M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 14, 215 (2013).
Huang, T., Chen, W., Liu, J., Gu, N. & Zhang, R. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat. Struct. Mol. Biol. 26, 380–388 (2019).
Delatte, B. et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 351, 282–285 (2016).
Safra, M. et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551, 251–255 (2017).
Li, X. et al. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell 68, 993–1005 (2017).
Marchand, V. et al. AlkAniline-Seq: profiling of m7G and m3C RNA modifications at single nucleotide resolution. Angew. Chem. Int. Ed. 57, 16785–16790 (2018).
Arango, D. et al. Acetylation of cytidine in mrna promotes translation efficiency. Cell 175, 1872–1886 (2018).
Sas-Chen, A. et al. Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping. Nature 583, 638–643 (2020).
Zhang, L.-S. et al. Transcriptome-wide mapping of internal N7-methylguanosine methylome in mammalian mRNA. Mol. Cell 74, 1304–1316.e8 (2019).
Pandolfini, L. et al. METTL1 promotes let-7 microRNA processing via m7G methylation. Mol. Cell 74, 1278–1290 (2019).
Delaunay, S. & Frye, M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 21, 552–559 (2019).
Haussmann, I. U. et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304 (2016).
Vu, L. P. et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 23, 1369–1376 (2017).
Novoa, E. M., Mason, C. E. & Mattick, J. S. Charting the unknown epitranscriptome. Nat. Rev. Mol. Cell Biol. 18, 339–340 (2017).
Anreiter, I., Mir, Q., Simpson, J. T., Janga, S. C. & Soller, M. New twists in detecting mRNA modification dynamics. Trends Biotechnol. 39, 72–89 (2020).
Li, X., Xiong, X. & Yi, C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat. Methods 14, 23–31 (2016).
Motorin, Y. & Helm, M. Methods for RNA modification mapping using deep sequencing: established and new emerging technologies. Genes 10, 35 (2019).
Grozhik, A. V. et al. Antibody cross-reactivity accounts for widespread appearance of m1A in 5′UTRs. Nat. Commun. 10, 1–13 (2019).
Lahens, N. F. et al. IVT-seq reveals extreme bias in RNA sequencing. Genome Biol. 15, R86 (2014).
Garalde, D. R. et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018).
Jonkhout, N. et al. The RNA modification landscape in human disease. RNA 23, 1754–1769 (2017).
Leger, A., Amaral, P. P., Pandolfini, L. & Capitanchik, C. RNA modifications detection by comparative Nanopore direct RNA sequencing. Preprint at bioRxiv https://doi.org/10.1101/843136 (2019).
Liu, H. et al. Accurate detection of m6A RNA modifications in native RNA sequences. Nat. Commun. 10, 4079 (2019).
Parker, M. T. et al. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. eLife 9, e49658 (2020).
Price, A. M. et al. Direct RNA sequencing reveals m6A modifications on adenovirus RNA are necessary for efficient splicing. Nat. Commun. 11, 6016 (2020).
Jenjaroenpun, P. et al. Decoding the epitranscriptional landscape from native RNA sequences. Nucleic Acids Res. 49, e7 (2020).
Lorenz, D. A., Sathe, S., Einstein, J. M. & Yeo, G. W. Direct RNA sequencing enables m6A detection in endogenous transcript isoforms at base-specific resolution. RNA 26, 19–28 (2020).
Pratanwanich, P. N. et al. Detection of differential RNA modifications from direct RNA sequencing of human cell lines. Preprint at bioRxiv https://doi.org/10.1101/2020.06.18.160010 (2020).
Jack, K. et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol. Cell 44, 660–666 (2011).
Yoon, A. et al. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science 312, 902–906 (2006).
Bellodi, C. et al. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. 70, 6026–6035 (2010).
Wu, G., Xiao, M., Yang, C. & Yu, Y.-T. U2 snRNA is inducibly pseudouridylated at novel sites by Pus7p and snR81 RNP. EMBO J. 30, 79–89 (2011).
Taoka, M., Nobe, Y., Hori, M. & Takeuchi, A. A mass spectrometry-based method for comprehensive quantitative determination of post-transcriptional RNA modifications: the complete chemical structure of Schizosaccharomyces pombe ribosomal RNAs. Nucleic Acids Res. 43, e115 (2015).
Basu, A. et al. Requirement of rRNA methylation for 80S ribosome assembly on a cohort of cellular internal ribosome entry sites. Mol. Cell. Biol. 31, 4482–4499 (2011).
Marcel, V. et al. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell 24, 318–330 (2013).
Belin, S. et al. Dysregulation of ribosome biogenesis and translational capacity is associated with tumor progression of human breast cancer cells. PLoS ONE 4, e7147 (2009).
Buchhaupt, M. et al. Partial methylation at Am100 in 18S rRNA of baker’s yeast reveals ribosome heterogeneity on the level of eukaryotic rRNA modification. PLoS ONE 9, e89640 (2014).
Chen, H. et al. METTL5, an 18S rRNA-specific m6A methyltransferase, modulates expression of stress response genes. Preprint at bioRxiv https://doi.org/10.1101/2020.04.27.064162 (2020).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Sović, I. et al. Fast and sensitive mapping of nanopore sequencing reads with GraphMap. Nat. Commun. 7, 11307 (2016).
Liu, Q. et al. Detection of DNA base modifications by deep recurrent neural network on Oxford Nanopore sequencing data. Nat. Commun. 10, 2449 (2019).
McIntyre, A. B. R. et al. Single-molecule sequencing detection of N6-methyladenine in microbial reference materials. Nat. Commun. 10, 579 (2019).
De Coster, W., Stovner, E. B. & Strazisar, M. Methplotlib: analysis of modified nucleotides from nanopore sequencing. Bioinformatics 36, 3236–3238 (2020).
Stoiber, M. et al. De novo identification of DNA modifications enabled by genome-guided nanopore signal processing. Preprint at bioRxiv https://doi.org/10.1101/094672 (2017).
Taoka, M. et al. The complete chemical structure of Saccharomyces cerevisiae rRNA: partial pseudouridylation of U2345 in 25S rRNA by snoRNA snR9. Nucleic Acids Res. 44, 8951–8961 (2016).
Pintard, L., Bujnicki, J. M., Lapeyre, B. & Bonnerot, C. MRM2 encodes a novel yeast mitochondrial 21S rRNA methyltransferase. EMBO J. 21, 1139–1147 (2002).
Sharma, S. & Lafontaine, D. L. J. ‘View from a bridge’: a new perspective on eukaryotic rRNA base modification. Trends Biochem. Sci 40, 560–575 (2015).
Taoka, M. et al. Landscape of the complete RNA chemical modifications in the human 80S ribosome. Nucleic Acids Res. 46, 9289–9298 (2018).
Fischer, N. et al. Structure of the E. coli ribosome–EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM. Nature 520, 567–570 (2015).
Sergeeva, O. V., Bogdanov, A. A. & Sergiev, P. V. What do we know about ribosomal RNA methylation in Escherichia coli? Biochimie 117, 110–118 (2015).
Sloan, K. E. et al. Tuning the ribosome: the influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol. 14, 1138–1152 (2017).
Hebras, J., Krogh, N., Marty, V., Nielsen, H. & Cavaillé, J. Developmental changes of rRNA ribose methylations in the mouse. RNA Biol. 17, 150–164 (2020).
Higa-Nakamine, S. et al. Loss of ribosomal RNA modification causes developmental defects in zebrafish. Nucleic Acids Res. 40, 391–398 (2012).
Sahoo, T. et al. Prader–Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat. Genet. 40, 719–721 (2008).
Heiss, N. S. et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 19, 32–38 (1998).
Knight, S. W. et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am. J. Hum. Genet. 65, 50–58 (1999).
Liao, J. et al. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Mol. Cancer 9, 198 (2010).
Mei, Y.-P. et al. Small nucleolar RNA 42 acts as an oncogene in lung tumorigenesis. Oncogene 31, 2794–2804 (2012).
Bortolin-Cavaille, M.-L., Bortolin-Cavaille, M. & Cavaille, J. The SNORD115 (H/MBII-52) and SNORD116 (H/MBII-85) gene clusters at the imprinted Prader–Willi locus generate canonical box C/D snoRNAs. Nucleic Acids Res. 40, 6800–6807 (2012).
Erales, J. et al. Evidence for rRNA 2′-O-methylation plasticity: control of intrinsic translational capabilities of human ribosomes. Proc. Natl Acad. Sci. USA 114, 12934–12939 (2017).
Krogh, N. et al. Profiling of 2′-O-Me in human rRNA reveals a subset of fractionally modified positions and provides evidence for ribosome heterogeneity. Nucleic Acids Res. 44, 7884–7895 (2016).
Birkedal, U. et al. Profiling of ribose methylations in RNA by high-throughput sequencing. Angew. Chem. Int. Ed. Engl. 54, 451–455 (2015).
Natchiar, S. K., Myasnikov, A. G., Kratzat, H., Hazemann, I. & Klaholz, B. P. Visualization of chemical modifications in the human 80S ribosome structure. Nature 551, 472–477 (2017).
van der Feltz, C., DeHaven, A. C. & Hoskins, A. A. Stress-induced pseudouridylation alters the structural equilibrium of yeast U2 snRNA stem II. J. Mol. Biol. 430, 524–536 (2018).
Frye, M., Harada, B. T., Behm, M. & He, C. RNA modifications modulate gene expression during development. Science 361, 1346–1349 (2018).
Roundtree, I. A., Evans, M. E., Pan, T. & He, C. Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017).
Li, S. & Mason, C. E. The pivotal regulatory landscape of RNA modifications. Annu. Rev. Genomics Hum. Genet. 15, 127–150 (2014).
Wang, X. et al. LARP7-mediated U6 snRNA modification ensures splicing fidelity and spermatogenesis in mice. Mol. Cell 77, 999–1013 (2020).
Louloupi, A., Ntini, E., Conrad, T. & Ørom, U. A. V. Transient N-6-methyladenosine transcriptome sequencing reveals a regulatory role of m6A in splicing efficiency. Cell Rep. 23, 3429–3437 (2018).
Zhou, K. I. et al. Regulation of co-transcriptional pre-mRNA splicing by m6A through the low-complexity protein hnRNPG. Mol. Cell 76, 70–81 (2019).
Lee, Y., Choe, J., Park, O. H. & Kim, Y. K. Molecular mechanisms driving mRNA degradation by m6A modification. Trends Genet. 36, 177–188 (2020).
Guo, M., Liu, X., Zheng, X., Huang, Y. & Chen, X. m6A RNA modification determines cell fate by regulating mRNA degradation. Cell. Reprogram. 19, 225–231 (2017).
Geula, S. et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006 (2015).
Weng, H. et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell 22, 191–205 (2018).
Lence, T. et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247 (2016).
Kan, L. et al. The m6A pathway facilitates sex determination in Drosophila. Nat. Commun. 8, 15737 (2017).
Schaefer, M., Kapoor, U. & Jantsch, M. F. Understanding RNA modifications: the promises and technological bottlenecks of the ‘epitranscriptome’. Open Biol. 7, 170077 (2017).
Depledge, D. P. et al. Direct RNA sequencing on nanopore arrays redefines the transcriptional complexity of a viral pathogen. Nat. Commun. 10, 754 (2019).
Roach, N. P. et al. The full-length transcriptome of C. elegans using direct RNA sequencing. Genome Res. 30, 299–312 (2020).
Workman, R. E. et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat. Methods 16, 1297–1305 (2019).
Kim, D. et al. The architecture of SARS-CoV-2 transcriptome. Cell 181, 914–921 (2020).
Parker, S. et al. A resource for functional profiling of noncoding RNA in the yeast Saccharomyces cerevisiae. RNA 23, 1166–1171 (2017).
Smith, M. A. et al. Molecular barcoding of native RNAs using nanopore sequencing and deep learning. Genome Res. 30, 1345–1353 (2020).
Li, H. et al. The sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Loman, N. J., Quick, J. & Simpson, J. T. A complete bacterial genome assembled de novo using only nanopore sequencing data. Nat. Methods 12, 733–735 (2015).
Acknowledgements
We thank all the members of the Novoa lab for their valuable insights and discussion. We thank V. Malhotra for sharing the Pus1 and Pus4 knockout strains. O.B. is supported by a University of New South Wales International PhD Fellowship. M.C.L. is supported by an FPI Severo-Ochoa Fellowship by the Spanish Ministry of Economy, Industry and Competitiveness (MEIC). I.M. and S.C. are supported by ‘la Caixa’ INPhINIT PhD Fellowships (LCF/BQ/DI18/11660028 and LCF/BQ/DI19/11730036, respectively). This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Marie Skodowska-Curie grant agreement no. 713673. This work was supported by the Australian Research Council (DP180103571 to E.M.N.) and the MEIC (PGC2018-098152-A-100 to E.M.N.). We acknowledge the support of the MEIC to the EMBL partnership, the Centro de Excelencia Severo Ochoa and the CERCA Programme/Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
O.B. and M.C.L. performed most wet lab experiments, including RNA extraction and nanopore library preparation. O.B. and L.P.P. performed bioinformatic analysis of the data, together with J.M.R. and E.M.N. O.B. conceived and performed nanoCMC-seq experiments. M.C.L. produced the in vitro transcribed sequences with modifications and their corresponding nanopore libraries. O.B. prepared and sequenced the in vitro transcribed sequences with different pseudouridine stoichiometries. L.P.P. benchmarked and wrote the nanoRMS code, together with O.B. and E.M.N. J.M.R. performed bioinformatic analyses on in vitro transcribed constructs and compared base-calling and mapping algorithms. I.M. built polysome gradients and helped with their corresponding nanopore libraries. S.C. and I.M. prepared and sequenced the 2′-O-methylation mutant strains. R.M. and H.G.S.V. cultured the S. cerevisiae strains under different stress conditions. H.L. contributed with code for the analysis of current intensity values. A.S.C. and S.S. cultured all snoRNA-depleted yeast mutant strains and extracted their total RNA. E.M.N. conceived the project. E.M.N. supervised the work, with the assistance of J.S.M. M.C.L., O.B. and E.M.N. built the figures. O.B., M.C.L. and E.M.N. wrote the paper, with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
E.M.N. has received travel and accommodation expenses to speak at Oxford Nanopore Technologies conferences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Peer review information Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Begik, O., Lucas, M.C., Pryszcz, L.P. et al. Quantitative profiling of pseudouridylation dynamics in native RNAs with nanopore sequencing. Nat Biotechnol 39, 1278–1291 (2021). https://doi.org/10.1038/s41587-021-00915-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-021-00915-6
This article is cited by
-
Single-molecule epitranscriptomic analysis of full-length HIV-1 RNAs reveals functional roles of site-specific m6As
Nature Microbiology (2024)
-
Detection of ribonucleotides embedded in DNA by Nanopore sequencing
Communications Biology (2024)
-
The lncRNA Snhg11, a new candidate contributing to neurogenesis, plasticity, and memory deficits in Down syndrome
Molecular Psychiatry (2024)
-
Exploring tRNAs and their modifications and crosstalk using Nano-tRNAseq
Nature Biotechnology (2024)
-
Quantitative analysis of tRNA abundance and modifications by nanopore RNA sequencing
Nature Biotechnology (2024)