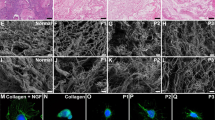
Abstract
Bioengineered uterine tissue could provide a treatment option for women with uterine factor infertility. In large animal models, reconstruction of the uterus has been demonstrated only with xenogeneic tissue grafts. Here we use biodegradable polymer scaffolds seeded with autologous cells to restore uterine structure and function in rabbits. Rabbits underwent a subtotal uterine excision and were reconstructed with autologous cell-seeded constructs, with nonseeded scaffolds or by suturing. At 6 months postimplantation, only the cell-seeded engineered uteri developed native tissue-like structures, including organized luminal/glandular epithelium, stroma, vascularized mucosa and two-layered myometrium. Only rabbits with cell-seeded constructs had normal pregnancies (four in ten) in the reconstructed segment of the uterus and supported fetal development to term and live birth. With further development, this approach may provide a regenerative medicine solution to uterine factor infertility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that supports the findings of this study are available from the corresponding author, upon reasonable request.
References
Taylor, E. & Gomel, V. The uterus and fertility. Fertil. Steril. 89, 1–16 (2008).
Chan, Y. Y. et al. Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound Obstet. Gynecol. 38, 371–382 (2011).
Abrao, M. S., Muzii, L. & Marana, R. Anatomical causes of female infertility and their management. Int. J. Gynaecol. Obst. 123, S18–S24 (2013).
2015 Assisted Reproductive Technology National Summary Report https://http://www.cdc.gov/art/pdf/2015-report/ART-2015-National-Summary-Report.pdf (Centers for Disease Control and Prevention, 2017).
Fageeh, W., Raffa, H., Jabbad, H. & Marzouki, A. Transplantation of the human uterus. Int. J. Gynaecol. Obstet. 76, 245–251 (2002).
Ozkan, O. et al. Preliminary results of the first human uterus transplantation from a multiorgan donor. Fertil. Steril. 99, 470–476 (2013).
Brannstrom, M. et al. The first clinical uterus transplantation trial: a six-month report. Fertil. Steril. 101, https://doi.org/10.1016/j.fertnstert.2014.02.024 (2014).
Brannstrom, M. et al. Livebirth after uterus transplantation. Lancet 385, 607–616 (2015).
Testa, G. et al. First live birth after uterus transplantation in the United States. Am. J. Transplant. 18, 1270–1274 (2018).
Baptista, P. M. & Atala, A. Regenerative medicine: the hurdles and hopes. Trans. Res. 163, 255–258 (2014).
Atala, A., Bauer, S. B., Soker, S., Yoo, J. J. & Retik, A. B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367, 1241–1246 (2006).
L’Heureux, N., McAllister, T. N. & de la Fuente, L. M. Tissue-engineered blood vessel for adult arterial revascularization. N. Engl. J. Med. 357, 1451–1453 (2007).
Olausson, M. et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet 380, 230–237 (2012).
Raya-Rivera, A. et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377, 1175–1182 (2011).
Raya-Rivera, A. M. et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 384, 329–336 (2014).
Campo, H., Cervello, I. & Simon, C. Bioengineering the uterus: an overview of recent advances and future perspectives in reproductive medicine. Ann. Biomed. Eng. 45, 1710–1717 (2017).
Hellstrom, M., Bandstein, S. & Brannstrom, M. Uterine tissue engineering and the future of uterus transplantation. Ann. Biomed. Eng. 45, 1718–1730 (2017).
Ansari, A. H., Gould, K. & Turner, R. J. Segmental uterine horn replacement in rabbit using umbilical vein. Obst. Gynecol. 60, 733–737 (1982).
Taveau, J. W. et al. Regeneration of uterine horn using porcine small intestinal submucosa grafts in rabbits. J. Invest. Surg. 17, 81–92 (2004).
De Filippo, R. E., Yoo, J. J. & Atala, A. Urethral replacement using cell seeded tubularized collagen matrices. J. Urol. 168, 1789–1792 (2002).
Dorin, R. P., Pohl, H. G., De Filippo, R. E., Yoo, J. J. & Atala, A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J. Urol 26, 323–326 (2008).
Fischer, B., Chavatte-Palmer, P., Viebahn, C., Navarrete Santos, A. & Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 144, 1–10 (2012).
Atala, A. et al. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J. Urol. 148, 658–662 (1992).
Oberpenning, F., Meng, J., Yoo, J. J. & Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 17, 149–155 (1999).
De Filippo, R. E., Yoo, J. J. & Atala, A. Engineering of vaginal tissue in vivo. Tissue Eng. 9, 301–306 (2003).
Chen, K. L., Eberli, D., Yoo, J. J. & Atala, A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc. Natl Acad. Sci. USA 107, 3346–3350 (2010).
Atala, A. Engineering tissues, organs and cells. J. Tissue Eng. Regen. Med. 1, 83–96 (2007).
Park, K. R. et al. CD9 is expressed on human endometrial epithelial cells in association with integrins alpha(6), alpha(3) and beta(1). Mol. Human Reprod. 6, 252–257 (2000).
Cervello, I. et al. Reconstruction of endometrium from human endometrial side population cell lines. PloS ONE 6, e21221 (2011).
Mosher, A. A. et al. Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth 13, S7 (2013).
Wooding, F. B. G. Comparative Olacentation: Structures, Functions, and Evolution 185–231 (Springer, 2008).
Jonkman, M. F., Kauer, F. M., Nieuwenhuis, P. & Molenaar, I. Segmental uterine horn replacement in the rat using a biodegradable microporous synthetic tube. Artificial Organs 10, 475–480 (1986).
Santoso, E. G. et al. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PloS ONE 9, e103201.
Campbell, G. R. et al. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J. Tissue Eng. Regen. Med. 2, 50–60 (2014).
Li, X. et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials 32, 8172–8181 (2011).
Ding, L. et al. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 35, 4888–4900 (2014).
Song, T. et al. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng. A. 21, 353–361 (2015).
Miyazaki, K. & Maruyama, T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35, 8791–8800 (2014).
Hellstrom, M. et al. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 106, https://doi.org/10.1016/j.fertnstert.2016.03.048 (2016).
Acknowledgements
This work was supported by grants from the National Institutes of Health (no. T32-EB014836-05) and the State of North Carolina. We thank J. Kassis for editorial assistance, T. Wang, H.G. Huddlestone and C. Bishop for their initial background contribution to the project and S. Lankford, A.S. Dean, I. Vasutin, T.J. Cockerham, M. Seeds and E.E. Whitaker for technical assistance.
Author information
Authors and Affiliations
Contributions
A.A. developed the concept of engineering functional autologous uterine tissue constructs. R.S.M., J.K.W., J.J.Y. and A.A. designed all experiments. R.S.M. performed the in vitro experiments and fabricated the bioengineered uterine constructs. R.S.M. and J.K.W. performed in vivo experiments of uterine tissue excision and construct implantation. K.W.Y. performed in vitro experiments and analyzed the data. R.S.M., J.K.W. and A.A. analyzed the data and wrote the manuscript. A.A. provided direction and supervised the project. R.S.M., J.K.W., J.J.Y. and A.A. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Boston Children’s Hospital was assigned the rights to two issued patents, both titled ‘Tissue Engineered Uterus’, no. 7,049,057, filed 15 November 2002, and no. 7,429,490, filed 7 February 2005, with A.A. and J.J.Y. listed as inventors. Based on the remaining patent term and the need for further studies before this technology is used clinically, there are no current or expected financial interests related to these patents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Rights and permissions
About this article
Cite this article
Magalhaes, R.S., Williams, J.K., Yoo, K.W. et al. A tissue-engineered uterus supports live births in rabbits. Nat Biotechnol 38, 1280–1287 (2020). https://doi.org/10.1038/s41587-020-0547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-020-0547-7
This article is cited by
-
New Solutions for Old Problems: How Reproductive Tissue Engineering Has Been Revolutionizing Reproductive Medicine
Annals of Biomedical Engineering (2023)
-
Regenerative Surgery: Is This an Independent Field of Health Sciences or Only a Semantic Exercise?
Current Transplantation Reports (2023)
-
Progress in Preclinical Research on Uterus Bioengineering That Utilizes Scaffolds Derived from Decellularized Uterine Tissue
Biomedical Materials & Devices (2023)
-
Design and Characterization of Maltose-Conjugated Polycaprolactone Nanofibrous Scaffolds for Uterine Tissue Engineering
Regenerative Engineering and Translational Medicine (2022)
-
Building a stem cell-based primate uterus
Communications Biology (2021)