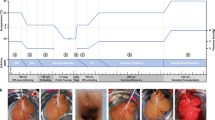
Abstract
The inability to preserve vascular organs beyond several hours contributes to the scarcity of organs for transplantation1,2. Standard hypothermic preservation at +4 °C (refs. 1,3) limits liver preservation to less than 12 h. Our group previously showed that supercooled ice-free storage at –6 °C can extend viable preservation of rat livers4,5 However, scaling supercooling preservation to human organs is intrinsically limited because of volume-dependent stochastic ice formation. Here, we describe an improved supercooling protocol that averts freezing of human livers by minimizing favorable sites of ice nucleation and homogeneous preconditioning with protective agents during machine perfusion. We show that human livers can be stored at –4 °C with supercooling followed by subnormothermic machine perfusion, effectively extending the ex vivo life of the organ by 27 h. We show that viability of livers before and after supercooling is unchanged, and that after supercooling livers can withstand the stress of simulated transplantation by ex vivo normothermic reperfusion with blood.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Any additional data if needed will be provided upon reasonable request.
References
Giwa, S. et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 35, 530–542 (2017).
Buying time for transplants. Nat. Biotechnol. 35, 801 (2017).
Bruinsma, B. G. & Uygun, K. Subzero organ preservation: the dawn of a new ice age? Curr. Opin. Organ Transplant. 22, 281–286 (2017).
Berendsen, T. A. et al. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 20, 790–793 (2014).
Bruinsma, B. G. et al. Supercooling preservation and transplantation of the rat liver. Nat. Protoc. 10, 484–494 (2015).
Huang, H., Yarmush, M. L. & Usta, O. B. Long-term deep-supercooling of large-volume water and red cell suspensions via surface sealing with immiscible liquids. Nat. Commun. 9, 3201 (2018).
Storey, K. B. & Storey, J. M. Molecular biology of freezing tolerance. Compr. Physiol. 3, 1283–1308.
Dutheil, D., Underhaug Gjerde, A., Petit-Paris, I., Mauco, G. & Holmsen, H. Polyethylene glycols interact with membrane glycerophospholipids: is this part of their mechanism for hypothermic graft protection? J. Chem. Biol. 2, 39–49 (2009).
Jacobsen, I. A., Pegg, D. E., Wusteman, M. C. & Robinson, S. M. Transplantation of rabbit kidneys perfused with glycerol solutions at 10 degrees C. Cryobiology 15, 18–26 (1978).
‘t Hart, N. A. et al. Determination of an adequate perfusion pressure for continuous dual vessel hypothermic machine perfusion of the rat liver. Transpl. Int. 20, 343–352 (2007).
Bruinsma, B. G. et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation: subnormothermic machine perfusion of human livers. Am. J. Transplant. 14, 1400–1409 (2014).
Bruinsma, B. G. et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 6, 22415 (2016).
Sridharan, G. V. et al. Metabolomic modularity analysis (MMA) to quantify human liver perfusion dynamics. Metabolites 7, 58 (2017).
Vajdová, K., Graf, R. & Clavien, P.-A. ATP-supplies in the cold-preserved liver: a long-neglected factor of organ viability. Hepatology 36, 1543–1552 (2002).
Higashi, H., Takenaka, K., Fukuzawa, K., Yoshida, Y. & Sugimachi, K. Restoration of ATP contents in the transplanted liver closely relates to graft viability in dogs. Eur. Surg. Res. 21, 76–82 (1989).
Bruinsma, B. G. et al. Peritransplant energy changes and their correlation to outcome after human liver transplantation. Transplantation 101, 1637–1644 (2017).
Lanir, A. et al. Hepatic transplantation survival: correlation with adenine nucleotide level in donor liver. Hepatology 8, 471–475 (1988).
Kamiike, W. et al. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation 45, 138–143 (1988).
Bruinsma, B. G., Berendsen, T. A., Izamis, M.-L., Yarmush, M. L. & Uygun, K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int. J. Artif. Organs 36, 775–780 (2013).
op den Dries, S. et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers: normothermic perfusion of human livers. Am. J. Transplant. 13, 1327–1335 (2013).
Sutton, M. E. et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PloS ONE 9, e110642 (2014).
Watson, C. J. E. et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. https://doi.org/10.1111/ajt.14687 (2018).
Avruch, J. H. et al. A novel model for ex situ reperfusion of the human liver following subnormothermic machine perfusion. Technology 05, 196–200 (2017).
Bral, M. et al. Preliminary single-center Canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am. J. Transplant. 17, 1071–1080 (2017).
Mergental, H. et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am. J. Transplant. 16, 3235–3245 (2016).
Matton, A. P. M. et al. Biliary bicarbonate, pH and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation https://journals.lww.com/transplantjournal/fulltext/2019/07000/Biliary_Bicarbonate,_pH,_and_Glucose_Are_Suitable.21.aspx (2018).
Reiling, J. et al. Urea production during normothermic machine perfusion: price of success? Liver Transpl. 21, 700–703 (2015).
Westerkamp, A. C. et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation 100, 825–835 (2016).
Borghi-Scoazec, G. et al. Apoptosis after ischemia-reperfusion in human liver allografts. Liver Transpl. Surg. 3, 407–415 (1997).
Manuchehrabadi, N. et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 9, eaah4586 (2017).
Acknowledgements
Funding from the US National Institutes of Health (NIH) (grant nos. R01DK096075, R01DK107875 and R01DK114506) and the Department of Defense (contract no. RTRP W81XWH-17-1-0680) are gratefully acknowledged. We thank Sylvatica Biotech, Inc. and are grateful for support through the NIH (grant no. R21EB023031) and the US Army MRDC (contract no. W81XWH-16-C-0067). R.J.V. acknowledges a stipend from the Michael van Vloten Fund for Surgical Research. S.N.T. acknowledges support from NIH grant no. K99 HL143149. We thank M. Karabacak, Y.M. Yu and F. Lin at the Mass Spectrometry Core Facility (Shriners Hospital for Children, Boston, MA) for assistance with adenylate quantification. We thank L. Burlage, A. Matton, B. Bruinsma and C. Pendexter for experimental assistance. Finally, appreciation is extended to LiveOnNY, and we are especially grateful for our collaboration with New England Donor Services and their generous support that enables research with human donor organs. The views, opinions and/or findings contained in this manuscript are those of the authors and should not be construed as an official position, policy or decision of any of the institutions that supported the research, unless so designated by other documentation.
Author information
Authors and Affiliations
Contributions
R.J.V., S.N.T. and K.U. conceived and designed the study. R.J.V., P.D.B., S.N., S.E.J.C. and S.O. performed data acquisition. R.J.V., S.N.T., E.O.A.H., H.Y., M.L.Y., J.F.M., M.T. and K.U. analyzed and interpreted data. R.J.V., S.N.T. and S.O. designed and constructed the perfusion and supercooling system. R.J.V., S.N.T. and K.U. wrote the manuscript. R.J.V., S.N.T., E.O.A.H., T.M.G., M.L.Y., J.F.M., M.T., H.Y. and K.U. participated in critical revision of the manuscript for intellectual content. R.J.V., S.N.T. and K.U. performed statistical analysis. All authors contributed to the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing financial interests. M.T., M.L.Y., R.J.V., K.U. and S.N.T. have provisional patent applications relevant to this study. K.U. has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Authors’ interests are managed by MGH and Partners HealthCare in accordance with their conflict of interest policies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 First attempt at supercooling a human liver with the rat protocol.
Left: A liver after convetntional hypothermic preservation (HP) in University of Winsconsin solution (UW). Right: A liver frozen at –6ºC after ealier failed attempts to supercool, shown after recovery from the chiller.
Supplementary Figure 2 Melting point depression of the liver grafts.
The symbols of the dot plot overlay correspond to the unique independent biological replicates (n = 5) and match between Figs. 2 and 3; Supplementary Figs. 2, 4 and 5; and Supplementary Table 2. Left: melting point of the human livers preserved University of Wisconsin hypothermic preservation (HP) solution. Right: melting point of the same human livers preserved in our supercooling (SC) preservation solution. Star denotes statistical significance (p = 0.001, paired two-tailed student’s t tests).
Supplementary Figure 3 Photos of the livers during the supercooling protocol.
a) Liver during the cooling phase of subnormothermic machine perfusion (SNMP). (b) Liver submerged in the chiller basin during ice-free subzero supercooled storage. (c) Liver during reperfusion.
Supplementary Figure 4 Important ex vivo viability parameters and histology during pre- and post-supercooling subnormothermic machine perfusion.
(a) Tissue adenylate triphoshate (ATP) and adenylate monophosphate (AMP) ratio. Blue denotes pre-supercooling and green denotes post-supercooling throughout the figure. The symbols of the dot plot overlay correspond to the unique independent biological replicates (n = 5 throughout the figure) and match between Figs. 2 and 3; Supplementary Figs. 2, 4 and 5; and Supplementary Table 2. (b) Tissue adenylate triphosphate (ATP) and adenylate diphosphate (ADP) ratio. (c) Lactate clearance derived from in and outflow measurements. (d) Lactate concentration (top) and pH (below) of the arterial inflow. (e) Perfusate potassium concentrations. (f) Urea concentrations in the perfusate. Stars denote statistical significance (p < 0.05, repeated measures two-way ANOVA followed by the Sidak multiple comparisons test). Boxes: median and IQR. Whiskers: min and max. Error bars of line graphs: mean ± SEM.
Supplementary Figure 5 Important ex vivo viability parameters during simulated transplantation by normothermic blood reperfusion.
(a) Tissue adenylate triphosphate (ATP) and adenylate monophosphate (AMP) ratio. The symbols of the dot plot overlay correspond to the unique independent biological replicates (n = 3 throughout the figure) and match between Figs. 2 and 3; Supplementary Figs. 2, 4 and 5; and Supplementary Table 2. (b) Tissue adenylate triphosphate (ATP) and adenylate diphosphate (ADP) ratio. (c) Oxygen uptake. (d) Plasma urea concentrations. (e) Lactate clearance derived from in and outflow measurements. (f) Plasma potassium concentrations. Error bars: mean ± SEM.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Supplementary Tables 1–4
Rights and permissions
About this article
Cite this article
de Vries, R.J., Tessier, S.N., Banik, P.D. et al. Supercooling extends preservation time of human livers. Nat Biotechnol 37, 1131–1136 (2019). https://doi.org/10.1038/s41587-019-0223-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0223-y