Abstract
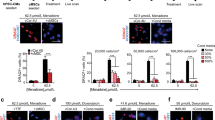
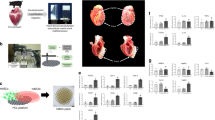
The epicardium and its derivatives provide trophic and structural support for the developing and adult heart. Here we tested the ability of human embryonic stem cell (hESC)-derived epicardium to augment the structure and function of engineered heart tissue in vitro and to improve efficacy of hESC-cardiomyocyte grafts in infarcted athymic rat hearts. Epicardial cells markedly enhanced the contractility, myofibril structure and calcium handling of human engineered heart tissues, while reducing passive stiffness compared with mesenchymal stromal cells. Transplanted epicardial cells formed persistent fibroblast grafts in infarcted hearts. Cotransplantation of hESC-derived epicardial cells and cardiomyocytes doubled graft cardiomyocyte proliferation rates in vivo, resulting in 2.6-fold greater cardiac graft size and simultaneously augmenting graft and host vascularization. Notably, cotransplantation improved systolic function compared with hearts receiving either cardiomyocytes alone, epicardial cells alone or vehicle. The ability of epicardial cells to enhance cardiac graft size and function makes them a promising adjuvant therapeutic for cardiac repair.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Braunwald, E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N. Engl. J. Med. 337, 1360–1369 (1997).
Mozaffarian, D. et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e360 (2016).
McMurray, J. J., Petrie, M. C., Murdoch, D. R. & Davie, A. P. Clinical epidemiology of heart failure: public and private health burden. Eur. Heart J. 19, P9–P16 (1998).
Bertero, A. & Murry, C. E. Hallmarks of cardiac regeneration. Nat. Rev. Cardiol. 15, 579–580 (2018).
Weinberger, F., Mannhardt, I. & Eschenhagen, T. Engineering cardiac muscle tissue: a maturating field of research. Circ. Res. 120, 1487–1500 (2017).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Meth. 11, 855–860 (2014).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA 109, E1848–E1857 (2012).
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007).
Patsch, C. et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 17, 994–1003 (2015).
Orlova, V. V. et al. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 9, 1514–1531 (2014).
Cheung, C., Bernardo, A. S., Trotter, M. W., Pedersen, R. A. & Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 30, 165–173 (2012).
Iyer, D. et al. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development 142, 1528–1541 (2015).
Witty, A. D. et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 32, 1026–1035 (2014).
Palpant, N. J. et al. Inhibition of beta-catenin signaling respecifies anterior-like endothelium into beating human cardiomyocytes. Development 142, 3198–3209 (2015).
Palpant, N. J. et al. Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat. Protoc. 12, 15–31 (2017).
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012).
Weinberger, F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 8, 363ra148 (2016).
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016).
Liu, Y. W. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605 (2018).
van den Berg, C. W. et al. Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development 142, 3231–3238 (2015).
Guadix, J. A., Carmona, R., Munoz-Chapuli, R. & Perez-Pomares, J. M. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev. Dyn. 235, 1014–1026 (2006).
Gittenberger-de Groot, A. C., Vrancken Peeters, M. P., Mentink, M. M., Gourdie, R. G. & Poelmann, R. E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 82, 1043–1052 (1998).
Dettman, R. W., Denetclaw, W. Jr., Ordahl, C. P. & Bristow, J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev. Biol. 193, 169–181 (1998).
Manner, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 255, 212–226 (1999).
Ieda, M. et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev. Cell 16, 233–244 (2009).
Eid, H. et al. Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ. Res. 71, 40–50 (1992).
Stuckmann, I., Evans, S. & Lassar, A. B. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev. Biol. 255, 334–349 (2003).
Weeke-Klimp, A. et al. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J. Mol. Cell. Cardiol. 49, 606–616 (2010).
Braitsch, C. M., Kanisicak, O., van Berlo, J. H., Molkentin, J. D. & Yutzey, K. E. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J. Mol. Cell. Cardiol. 65, 108–19 (2013).
Ruan, J. L. et al. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 134, 1557–1567 (2016).
Dubois, N. C. et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 29, 1011–1018 (2011).
Winter, E. M. et al. A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ. Heart Fail. 2, 643–653 (2009).
Gerbin, K. A., Yang, X., Murry, C. E. & Coulombe, K. L. Enhanced electrical integration of engineered human myocardium via intramyocardial versus epicardial delivery in infarcted rat hearts. PloS ONE 10, e0131446 (2015).
van Tuyn, J. et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 25, 271–278 (2007).
Bax, N. A. et al. Epithelial-to-mesenchymal transformation alters electrical conductivity of human epicardial cells. J. Cell. Mol. Med. 15, 2675–2683 (2011).
Kirby, M. L., Gale, T. F. & Stewart, D. E. Neural crest cells contribute to normal aorticopulmonary septation. Science 220, 1059–1061 (1983).
Porras, D. & Brown, C. B. Temporal-spatial ablation of neural crest in the mouse results in cardiovascular defects. Dev. Dyn. 237, 153–162 (2008).
Jiang, X., Rowitch, D. H., Soriano, P., McMahon, A. P. & Sucov, H. M. Fate of the mammalian cardiac neural crest. Development 127, 1607–1616 (2000).
Cai, C. L. et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 (2008).
Gittenberger-de Groot, A. C., Vrancken Peeters, M. P., Bergwerff, M., Mentink, M. M. & Poelmann, R. E. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ. Res. 87, 969–971 (2000).
Ogle, B. M. et al. Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med. 8, 342ps13 (2016).
Lepilina, A. et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619 (2006).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Wang, J., Karra, R., Dickson, A. L. & Poss, K. D. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 382, 427–435 (2013).
Winter, E. M. et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation 116, 917–927 (2007).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Bargehr, J. et al. Embryological origin of human smooth muscle cells influences their ability to support endothelial network formation. Stem Cells Transl. Med. 5, 946–959 (2016).
Hofsteen, P., Robitaille, A. M., Chapman, D. P., Moon, R. T. & Murry, C. E. Quantitative proteomics identify DAB2 as a cardiac developmental regulator that inhibits WNT/beta-catenin signaling. Proc. Natl Acad. Sci. USA 113, 1002–1007 (2016).
Palpant, N. J., Hofsteen, P., Pabon, L., Reinecke, H. & Murry, C. E. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PloS ONE 10, e0126259 (2015).
Young, J. L. & Engler, A. J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32, 1002–1009 (2011).
Ruan, J. L. et al. Mechanical stress promotes maturation of human myocardium from pluripotent stem cell-derived progenitors. Stem Cells 33, 2148–2157 (2015).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Barnett, D. W., Garrison, E. K., Quinlan, A. R., Stromberg, M. P. & Marth, G. T. BamTools: a C++ API and toolkit for analyzing and managing BAM files. Bioinformatics 27, 1691–1692 (2011).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Binns, D. et al. QuickGO: a web-based tool for Gene Ontology searching. Bioinformatics 25, 3045–3046 (2009).
Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl Acad. Sci. USA 95, 14863–14868 (1998).
Wang, J., Vasaikar, S., Shi, Z., Greer, M. & Zhang, B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 45, W130–W137 (2017).
Acknowledgements
This work was supported by the British Heart Foundation (BHF; grants nos. NH/11/1/28922, G1000847, FS/13/29/30024 and FS/18/46/33663), Oxford-Cambridge Centre for Regenerative Medicine (grant no. RM/13/3/30159), the UK Medical Research Council (MRC) and the Cambridge Hospitals National Institute for Health Research Biomedical Research Centre funding (S.S.), as well as National Institutes of Health grant nos. P01HL094374, P01GM081619 and R01HL128362 and a grant from the Fondation Leducq Transatlantic Network of Excellence (C.E.M.). J.B. was supported by a Cambridge National Institute for Health Research Biomedical Research Centre Cardiovascular Clinical Research Fellowship and, subsequently, by a BHF Studentship (grant no. FS/13/65/30441). D.I. received a University of Cambridge Commonwealth Scholarship. L.G. is supported by BHF Award RM/l3/3/30159 and L.P.O. is funded by a Wellcome Trust Fellowship (grant no. 203568/Z/16/Z). N.F. was supported by BHF grant no. RG/13/14/30314. N.L.N. was supported by the Biotechnology and Biological Sciences Research Council (Institute Strategic Programmes BBS/E/B/000C0419 and BBS/E/B/000C0434). S.S. and M.R.B. were supported by the BHF Centre for Cardiovascular Research Excellence. Core support was provided by the Wellcome-MRC Cambridge Stem Cell Institute (grant no. 203151/Z/16/Z). The authors thank Osiris for providing the primary mesenchymal stem cells47.
Author information
Authors and Affiliations
Contributions
J.B. was the principal experimentalist and was responsible for study design and conceptualization, data acquisition and interpretation, production of figures and manuscript writing. L.P.O. performed tissue culture, 3D-EHT generation, force measurement and assisted during surgery. M.C. performed tissue culture and 3D-EHT generation. H.D. performed force measurement and RT–qPCR. P.H. was responsible for preparation of cell suspensions on the day of transplantation, necropsy, assisting during surgery and postoperative animal care. S.B. performed the casting of 3D-EHT and force measurement. L.G. performed tissue histology, immunofluorescence and sample preparation for RNAseq. N.L.N. performed the bioinformatics analysis. D.I. contributed conceptual ideas and critically revised the manuscript for important intellectual content. F.S. critically revised the manuscript for important intellectual content. F.W. performed the functional analysis of echocardiographs. A.B. performed the gene expression analysis. A.L. was responsible for experimental guidance and force measurement data analysis. W.G.B. was responsible for data interpretation and logistics. A.M. was responsible for animal surgery and logistics. N.F. was responsible for processing of histologic tissue and preparation of slides. M.R. was responsible for force measurement equipment. M.R.B. critically revised the manuscript for important intellectual content. C.E.M. was responsible for study design and conception, obtaining research funding, study supervision, and editing and final approval of the manuscript. S.S. was responsible for study design and conception, obtaining research funding, study supervision, interpretation of data, and editing and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent has been filed on the cardiac application of epicardial cells, on which C.E.M., S.S. and J.B. are coinventors (WO2018170280A1). C.E.M. is a scientific founder and equity holder in Cytocardia.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20 and Supplementary Tables. 1–5
Rights and permissions
About this article
Cite this article
Bargehr, J., Ong, L.P., Colzani, M. et al. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat Biotechnol 37, 895–906 (2019). https://doi.org/10.1038/s41587-019-0197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0197-9
This article is cited by
-
Proteomic analysis of neonatal mouse hearts shows PKA functions as a cardiomyocyte replication regulator
Proteome Science (2023)
-
Identification of mesenchymal-to-epithelial transition during heart regeneration through genetic lineage tracing
Stem Cell Research & Therapy (2023)
-
Mature human induced pluripotent stem cell-derived cardiomyocytes promote angiogenesis through alpha-B crystallin
Stem Cell Research & Therapy (2023)
-
Transcription Factors and Splice Factors—Interconnected Regulators of Stem Cell Differentiation
Current Stem Cell Reports (2023)
-
Activation of a transient progenitor state in the epicardium is required for zebrafish heart regeneration
Nature Communications (2022)