Abstract
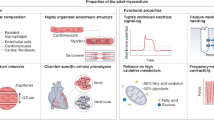
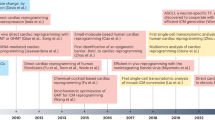
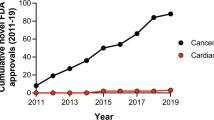
The retraction of >30 falsified studies by Anversa et al. has had a disheartening impact on the cardiac cell therapeutics field. The premise of heart muscle regeneration by the transdifferentiation of bone marrow cells or putative adult resident cardiac progenitors has been largely disproven. Over the past 18 years, a generation of physicians and scientists has lost years chasing these studies, and patients have been placed at risk with little scientific grounding. Funding agencies invested hundreds of millions of dollars in irreproducible work, and both academic institutions and the scientific community ignored troubling signals over a decade of questionable work. Our collective retrospective analysis identifies preventable problems at the level of the editorial and peer-review process, funding agencies and academic institutions. This Perspective provides a chronology of the forces that led to this scientific debacle, integrating direct knowledge of the process. We suggest a science-driven path forward that includes multiple novel approaches to the problem of heart muscle regeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Oransky, I. & Marcus, A. Harvard and the Brigham call for more than 30 retractions of cardiac stem cell research. STAT https://www.statnews.com/2018/10/14/harvard-brigham-retractions-stem-cell/ (2018).
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J. & Campbell, K. H. Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813 (1997).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Krause, D. S. et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105, 369–377 (2001).
Priller, J. et al. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 155, 733–738 (2001).
Corbel, S. Y. et al. Contribution of hematopoietic stem cells to skeletal muscle. Nat. Med. 9, 1528–1532 (2003).
Lagasse, E. et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6, 1229–1234 (2000).
LaBarge, M. A. & Blau, H. M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111, 589–601 (2002).
Alvarez-Dolado, M. et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973 (2003).
Weimann, J. M., Johansson, C. B., Trejo, A. & Blau, H. M. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 5, 959–966 (2003).
Morrison, S. J., Uchida, N. & Weissman, I. L. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11, 35–71 (1995).
Massengale, M., Wagers, A. J., Vogel, H. & Weissman, I. L. Hematopoietic cells maintain hematopoietic fates upon entering the brain. J. Exp. Med. 201, 1579–1589 (2005).
Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259 (2002).
Weimann, J. M., Charlton, C. A., Brazelton, T. R., Hackman, R. C. & Blau, H. M. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc. Natl Acad. Sci. USA 100, 2088–2093 (2003).
Bjornson, C. R., Rietze, R. L., Reynolds, B. A., Magli, M. C. & Vescovi, A. L. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283, 534–537 (1999).
Beltrami, A. P. et al. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 344, 1750–1757 (2001).
Leri, A. et al. Telomerase expression and activity are coupled with myocyte proliferation and preservation of telomeric length in the failing heart. Proc. Natl Acad. Sci. USA 98, 8626–8631 (2001).
Quaini, F. et al. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002).
Orlic, D. et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl Acad. Sci. USA 98, 10344–10349 (2001).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Strauer, B. E. et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913–1918 (2002).
Strauer, B. E. et al. [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction]. Dtsch Med. Wochenschr. 126, 932–938 (2001).
Wollert, K. C. et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148 (2004).
Assmus, B. et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 355, 1222–1232 (2006).
Murry, C. E. et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668 (2004).
Balsam, L. B. et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 428, 668–673 (2004).
Nygren, J. M. et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10, 494–501 (2004).
Chien, K. R. Stem cells: lost in translation. Nature 428, 607–608 (2004).
Laugwitz, K. L. et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433, 647–653 (2005).
Moretti, A. et al. Multipotent embryonic Isl1 + progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127, 1151–1165 (2006).
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008).
Bu, L. et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 460, 113–117 (2009).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Linke, A. et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl Acad. Sci. USA 102, 8966–8971 (2005).
Dawn, B. et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc. Natl Acad. Sci. USA 102, 3766–3771 (2005).
Hatzistergos, K. E. et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 107, 913–922 (2010).
Fischer, K. M. et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 120, 2077–2087 (2009).
Williams, A. R. et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127, 213–223 (2013).
Li, Q. et al. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 106, 849–864 (2011).
Oh, H. et al. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann. NY Acad. Sci. 1015, 182–189 (2004).
Smith, R. R. et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908 (2007).
Bolli, R. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011).
Makkar, R. R. et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012).
Pouly, J. et al. Cardiac stem cells in the real world. J. Thorac. Cardiovasc. Surg. 135, 673–678 (2008).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Soonpaa, M. H. & Field, L. J. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am. J. Physiol. 272, H220–H226 (1997).
Rumyantsev, P. P. DNA synthesis and nuclear division in embryonal and postnatal histogenesis of myocardium (autoradiographic study). Fed. Proc. Transl. Suppl. 24, 899–902 (1965).
van Berlo, J. H. et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014).
Moccetti, T., Leri, A. & Anversa, P. Controversy in myocardial regeneration. Regen. Med. 10, 921–924 (2015).
Sultana, N. et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat. Commun. 6, 8701 (2015).
Vagnozzi, R. J. et al. Genetic lineage tracing of Sca-1+ cells reveals endothelial but not myogenic contribution to the murine heart. Circulation 138, 2931–2939 (2018).
Neidig, L. E. et al. Evidence for minimal cardiogenic potential of stem cell antigen 1-positive cells in the adult mouse heart. Circulation 138, 2960–2962 (2018).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Kajstura, J. et al. Cardiomyogenesis in the aging and failing human heart. Circulation 126, 1869–1881 (2012); retraction 129, e466 (2014).
Wollert, K. C. et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 38, 2936–2943 (2017).
Janssens, S. et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367, 113–121 (2006).
Nowbar, A. N. et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. Br. Med. J. 348, g2688 (2014).
Morgan, P. et al. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat. Rev. Drug Discov. 17, 167–181 (2018).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA 109, E1848–E1857 (2012).
Birket, M. J. et al. Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells. Nat. Biotechnol. 33, 970–979 (2015).
Lee, J. H., Protze, S. I., Laksman, Z., Backx, P. H. & Keller, G. M. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell 21, 179–194.e4 (2017).
Foo, K. S. et al. Human ISL1+ ventricular progenitors self-assemble into an in vivo functional heart patch and preserve cardiac function post infarction. Mol. Ther. 26, 1644–1659 (2018).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Liu, Y. W. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 36, 597–605 (2018).
Menasché, P. et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 71, 429–438 (2018).
Sahara, M., Santoro, F. & Chien, K. R. Programming and reprogramming a human heart cell. EMBO J. 34, 710–738 (2015).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Mohamed, T. M. A. et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116.e12 (2018).
Acknowledgements
The authors gratefully acknowledge the careful editing and review of the manuscript by M. Sahara of the Karolinska Institutet.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
K.R.C. is a scientific founder and equity holder in Moderna Therapeutics and Procella Therapeutics, and chair of the External Science Panel for AstraZeneca. R.F.-D. is an employee of AstraZeneca. J.F. is an advisor to 10XGenomics. D.A.M. is cofounder of Semma Therapeutics. C.E.M. is a scientific founder and equity holder in Cytocardia. I.L.W. is a cofounder of, director of, stockholder in and consultant to Forty Seven Inc, a company currently devoted to cancer immunotherapies with antibodies to macrophage checkpoint inhibitors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chien, K.R., Frisén, J., Fritsche-Danielson, R. et al. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol 37, 232–237 (2019). https://doi.org/10.1038/s41587-019-0042-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0042-1
This article is cited by
-
Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway
BMC Medicine (2023)
-
Exercise Promotes Tissue Regeneration: Mechanisms Involved and Therapeutic Scope
Sports Medicine - Open (2023)
-
Development of a potency assay for CD34+ cell-based therapy
Scientific Reports (2023)
-
Pluripotent stem cell-derived committed cardiac progenitors remuscularize damaged ischemic hearts and improve their function in pigs
npj Regenerative Medicine (2023)
-
Molecular Circuit Discovery for Mechanobiology of Cardiovascular Disease
Regenerative Engineering and Translational Medicine (2023)