Abstract
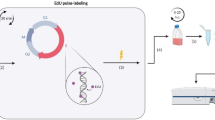
Selective differentiation of naive T cells into multipotent T cells is of great interest clinically for the generation of cell-based cancer immunotherapies. Cellular differentiation depends crucially on division state and time. Here we adapt a dye dilution assay for tracking cell proliferative history through mass cytometry and uncouple division, time and regulatory protein expression in single naive human T cells during their activation and expansion in a complex ex vivo milieu. Using 23 markers, we defined groups of proteins controlled predominantly by division state or time and found that undivided cells account for the majority of phenotypic diversity. We next built a map of cell state changes during naive T-cell expansion. By examining cell signaling on this map, we rationally selected ibrutinib, a BTK and ITK inhibitor, and administered it before T cell activation to direct differentiation toward a T stem cell memory (TSCM)-like phenotype. This method for tracing cell fate across division states and time can be broadly applied for directing cellular differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Code availability
An extended version of the Java-based Vortex software16 and documentation can be accessed at https://github.com/nolanlab/vortex.
Data availability
Single-cell data can be accessed at the Stanford Digital Repository (https://purl.stanford.edu/db057gb5997). Singe-cell RNA-sequencing data are also available on Gene Expression Omnibus (GEO accession code GSE119139).
References
Chang, J. T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007).
Gerlach, C. et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science 340, 635–639 (2013).
Tubo, N. J. et al. Most microbe-specific naïve CD4+ T cells produce memory cells during infection. Science 351, 511–514 (2016).
Reiner, S. L. & Adams, W. C. Lymphocyte fate specification as a deterministic but highly plastic process. Nat. Rev. Immunol. 14, 699–704 (2014).
Woodworth, M. B., Girskis, K. M. & Walsh, C. A. Building a lineage from single cells: genetic techniques for cell lineage tracking. Nat. Rev. Genet. 18, 230–244 (2017).
Quah, B. J. & Parish, C. R. New and improved methods for measuring lymphocyte proliferation in vitro and in vivo using CFSE-like fluorescent dyes. J. Immunol. Methods 379, 1–14 (2012).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Lyons, A. B. & Parish, C. R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171, 131–137 (1994).
Quah, B. J., Lyons, A. B. & Parish, C. R. The use of CFSE-like dyes for measuring lymphocyte proliferation: experimental considerations and biological variables. Math. Model. Nat. Phenom. 7, 53–64 (2012).
Li, Y. & Kurlander, R. J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl. Med. 8, 104 (2010).
Apetoh, L. et al. Consensus nomenclature for CD8+ T cell phenotypes in cancer. Oncoimmunology 4, e998538 (2015).
Gattinoni, L., Speiser, D. E., Lichterfeld, M. & Bonini, C. T memory stem cells in health and disease. Nat. Med. 23, 18–27 (2017).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Nish, S. A. et al. CD4+T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J. Exp. Med. 214, 39–47 (2017).
Jacomy, M., Venturini, T., Heymann, S. & Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One 9, e98679 (2014).
Samusik, N., Good, Z., Spitzer, M. H., Davis, K. L. & Nolan, G. P. Automated mapping of phenotype space with single-cell data. Nat. Methods 13, 493–496 (2016).
Zunder, E. R., Lujan, E., Goltsev, Y., Wernig, M. & Nolan, G. P. A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 16, 323–337 (2015).
Coifman, R. R. et al. Geometric diffusions as a tool for harmonic analysis and structure definition of data: diffusion maps. Proc. Natl Acad. Sci. USA 102, 7426–7431 (2005).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Series B Stat. Methodol. 58, 267–288 (1996).
Xu, L., Zhang, Y., Luo, G. & Li, Y. The roles of stem cell memory T cells in hematological malignancies. J. Hematol. Oncol. 8, 113 (2015).
Sabatino, M. et al. Generation of clinical-grade CD19-specific CAR-modified CD8+memory stem cells for the treatment of human B-cell malignancies. Blood 128, 519–528 (2016).
Dubovsky, J. A. et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122, 2539–2549 (2013).
Begum, J. et al. A method for evaluating the use of fluorescent dyes to track proliferation in cell lines by dye dilution. Cytometry A 83, 1085–1095 (2013).
Marchingo, J. M. et al. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 346, 1123–1127 (2014).
Salter, A. I. et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 11, eaat6753 (2018).
Kalia, V. et al. Prolonged interleukin-2Rα expression on virus-specific CD8+T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103 (2010).
Long, M. et al. Ibrutinib treatment improves T cell number and function in CLL patients. J. Clin. Invest. 127, 3052–3064 (2017).
Turtle, C. J. et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 35, 3010–3020 (2017).
Fraietta, J. A. et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127, 1117–1127 (2016).
Gill, S. et al. CD19 CAR-T cells combined with ibrutinib to induce complete remission in CLL. J. Clin. Oncol. 35(Suppl), 7509 (2017).
Quah, B.J. & Parish, C.R. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J. Vis. Exp. https://doi.org/10.3791/2259 (2010).
Advani, R. H. et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 31, 88–94 (2013).
Marostica, E. et al. Population pharmacokinetic model of ibrutinib, a Bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother. Pharmacol. 75, 111–121 (2015).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Stenton, S. B., Partovi, N. & Ensom, M. H. Sirolimus: the evidence for clinical pharmacokinetic monitoring. Clin. Pharmacokinet. 44, 769–786 (2005).
Fienberg, H. G., Simonds, E. F., Fantl, W. J., Nolan, G. P. & Bodenmiller, B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry A 81, 467–475 (2012).
Zunder, E. R. et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc. 10, 316–333 (2015).
Finck, R. et al. Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494 (2013).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Angerer, P. et al. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics 32, 1241–1243 (2016).
Bates, D., Machler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
James, G., Witten, D., Hastie, T. & Tibshirani, R. An Introduction to Statistical Learning with Applications in R 6th edn (Springer Texts in Statistics, New York, 2015).
Acknowledgements
We thank V. Gonzalez Munoz for panel design; J. Coller, D. Wagh (Stanford Functional Genomics Facility) and A. K. Shalek (UCSF) for single-cell RNA-seq advice; and S. Bhate, L. F. Yankielowicz-Keren, M. H. Spitzer, P. F. Gherardini and N. Agaheepour for insightful discussions. Z.G. was supported by the NIH training grants 5T32AI007290-29, -30, -32, -33 and 2T32AI007290-31. G.P.N. was supported by NIH grants R01CA184968, 1R01GM10983601, 1R01NS08953301, 1R01CA19665701, R01HL120724, 1R21CA183660, R33CA0183692, 1R33CA183654-01, U19AI057229, 1U19AI100627, U54-UCA149145A, N01-HV-00242 HHSN26820100034C and 5UH2AR067676; NIH Northrop-Grumman Corporation subcontract 7500108142; FDA grant HHSF223201210194C; DOD grants OC110674 and W81XWH-14-1-0180; the NWCRA Entertainment Industry Foundation; and Bill and Melinda Gates Foundation grant OPP1113682. Z.G., G.P.N. and S.C.B. are members of the Parker Institute for Cancer Immunotherapy, which supported this work. S.C.B. was supported by a Damon Runyon Cancer Research Foundation Fellowship (DRG-2017-09); NIH 1DP2OD022550-01, 1R01AG056287-01, 1R01AG057915-01, 1-R00-GM104148-01, 1U24CA224309-01, 5U19AI116484-02 and U19 AI104209; and a Translational Research Award from the Stanford Cancer Institute. The Illumina HiSeq 4000 used here was purchased with NIH funds (award S10OD018220).
Author information
Authors and Affiliations
Contributions
Z.G. and S.C.B. conceived the project and wrote the manuscript. Z.G. performed experiments and analyzed data. N.V.G. designed and carried out experiments on CFSE protocol optimization. L.B. designed and executed single-cell RNA-seq on prospectively isolated cell division states and assisted with initial antibody panel development. B.S. performed intracellular cytokine production assays and assisted with drug treatment experiments. N.S. implemented Vortex extensions. N.S. and R.T. advised on statistical and computational analyses. S.C.B. and G.P.N. secured funding and supervised the project. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Z.G., G.P.N. and S.C.B. are inventors on intellectual property applications for engineering T cells using methods described in this study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Establishing optimal conditions for detecting CFSE by mass cytometry.
(a-f) Human peripheral blood mononuclear cells (PBMCs) were stained with CFSE and an anti-FITC-172Yb antibody and analyzed by mass cytometry using an antibody panel in Supplementary Data 1. Tested deviations from the optimal staining conditions (see Methods) are indicated in each figure. Population of interest was selected either as CD8+ T cells (a-e), or myeloid dendritic cells (DCs) and monocytes (f). (a) Clones FIT-22 and F4/1 of anti-FITC antibody conjugated to the 172Yb reporter metal isotope can be used for detecting CFSE by mass cytometry. Anti-FITC-172Yb antibody titration identified optimal staining concentration as ≥16 µg/mL for both clones (red box). (b) Polyclonal anti-FITC-172Yb antibody as well as clones FIT-22 and F4/1 are compatible with both methanol and saponin permeabilization (standard intracellular antibody staining protocol uses methanol). Cells must be permeabilized to detect CFSE by mass cytometry. (c) Direct comparison of three anti-FITC-172Yb antibodies applied at chosen concentrations shows that the polyclonal antibody (red box) yields a superior signal even at 8 µg/mL compared to both clones FIT-22 and F4/1 at 16 µg/mL. (d) Polyclonal anti-FITC-172Yb antibody incubation extended from 30 to 60 minutes shows that even at 30 minutes (red box) the antibody binding is complete. (e) For optimal CFSE detection, cells must be alive during labeling, likely due to the requirement of esterases to cleave off acetate groups from the CFSE precursor CFDA-SE. (f) The quality of CFSE detection by mass cytometry is high even in cells with high autofluorescence, such as myeloid DCs and monocytes. Antibodies were titrated once; results in (b-e) are representative of 2 experiments; results in (f) are representative of 3 experiments.
Supplementary Figure 2 Comparison of cell proliferative history assessed by flow cytometry and mass cytometry.
(a) Spearman correlation analysis comparing CFSE signal (geometric mean) in primary human CD8+ T cells falling into each division state. Correlation test was applied to log10-transformed values. Time-series samples (n = 26 data points from 6 biological samples) were collected from the rapid expansion protocol (REP) cultures on days 0, 3, 4, 5, and 7 and grouped by day. Division IDs were assigned to the identical samples based on either flow cytometry or mass cytometry analysis. Days of the sample collection are indicated using a green color gradient. Red arrows indicate observed trends. (b) Flow cytometry and mass cytometry CFSE signal drift in samples from (a) grouped by division state. Linear regression was applied to CFSE signal from cells falling into the same division state. Slope is indicated next to each linear regression line. Division states are indicated using a green color gradient. (c) CFSE signal reduction per division was calculated based on geometric means within each sample from (a) at each day of collection. Boxplots show quartiles with a band at median, whiskers indicating 1.5 interquartile range (IQR), and outliers shown separately. A red dashed line indicates the expected 50% reduction. Results in (a-c) are from 1 experiment representative of 3 experiments and were obtained using an antibody panel in Supplementary Data 2.
Supplementary Figure 3 Phenotypic characterization of CFSE-traced naive CD8+ T cells.
(a) Complete gating strategy for CFSE-positive (naive) and CFSE-negative (remaining) T cells after magnetic enrichment analyzed by mass cytometry (REP day 0; see Fig. 1d for REP graphical summary). (b) Gating strategy demonstrating enrichment of naive phenotype among CFSE-positive vs. CFSE-negative CD8+ T cells from (a). (c) Expression of additional protein markers on CFSE-positive and CFSE-negative T cells gated as in (a). (d) A gating strategy demonstrating the utility of using CFSE labeling for tracking cells of interest in a high-parameter mass cytometry assay. T cells on REP day 0 from (a) are compared to day 4. (e) Features associated with differentiation are plotted for naive CD8+ T cells over 7 days of REP culture. Mean division IDs and relative cell numbers are included for reference. Relative protein expression is shown on relevant scales: mean arsinh-transformed expression for CD45RA and CD45RO, or percent positive cells for activation markers (CD25, CD69), marker of translational activity phosphorylated ribosomal protein S6 (pRPS6), and marker of proliferation Ki-67. Results in (a-b) and (d-e) are from 1 experiment representative of 3 experiments and were obtained using an antibody panel in Supplementary Data 2. Results in (c) are from 1 mass cytometry experiment utilizing an antibody panel in Supplementary Data 5.
Supplementary Figure 4 Division-state-dependent and division-state-independent single-cell protein expression during early differentiation of naive CD8+ T cells.
(a-b) Violin plots showing division state-dependent single-cell expression of T-cell activation markers (a) CD25 (IL2Rα) and (b) CD69. (c-d) Violin plots showing division state-dependent single-cell expression of T-cell differentiation markers (c) CD45RA and (d) CD45RO. (e-f) Violin plots showing division state-independent single-cell expression of inhibitory receptors (e) CD223 (LAG3) and (f) CD272 (BTLA). Gated on CFSE+ CD8+ T cells from the same REP culture on day 0 (naive), 3, 4, 5, and 7, either on bulk cells (gray; left) or split by division state (green gradient; right). Data were arsinh-transformed and normalized to an average of naive CD8+ T cells (black dotted line). Violin plots show a kernel density estimate with a boxplot, which contains quartiles with a band at median and whiskers indicating 1.5 IQR. Results are from 1 experiment representative of 3 experiments and were obtained using an antibody panel in Supplementary Data 2.
Supplementary Figure 5 Division-state-dependent and time-dependent changes in single-marker diversity during early differentiation of naive CD8+ T cells.
Division state-dependent only (left) and time-dependent only (right) changes in cellular diversity of 23 markers (plus CD3ε, CD8α, CD45 that were also used for gating) in naive CFSE+ CD8+ T cells (n = 6 biological samples) cultured via REP for 3, 4, 5, or 7 days. Cellular diversity with respect to each marker was estimated as standard deviation (s.d.) in arsinh-transformed individual marker expression. Data was normalized as shown in Fig. 2a. Results in are from 1 experiment representative of 3 experiments and were obtained using an antibody panel in Supplementary Data 2.
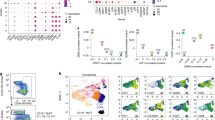
Supplementary Figure 6 Marker expression projected onto a force-directed graph showing naive CD8+ T-cell states on day 3 of expansion.
(a-b) A force-directed graph was constructed using expression of 21 surface proteins and contains 10,000 CFSE+ CD8+ T cells on day 3 of naive T-cell differentiation via REP. (a) Expression of 21 surface proteins. (b) Expression of 2 functional markers Ki-67 (proliferation) and pRPS6 (translational activity). CFSE is shown for a reference. Results were obtained using an antibody panel in Supplementary Data 2 and refer to data shown in Fig. 3b.
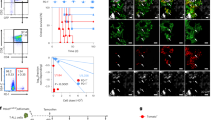
Supplementary Figure 7 Undivided cells occupy the majority of phenotypic niches on day 3 of naive T-cell expansion.
(a-b) A diffusion map was constructed using expression of 21 surface proteins listed in Fig. 3a and contains 10,000 CFSE+ CD8+ T cells on day 3 of naive T-cell expansion via REP. Cells were projected onto diffusion components 1-3 to highlight the continuity and endpoints of T-cell differentiations states. (a) Cells are shown separated by division state, demonstrating that undivided cells appear in the majority of phenotypic niches. (b) All cells (top) or undivided cells only (bottom) with division states (left), diffusion pseudo-time (DPT) (center), or expression of proteins associated with DPT1 (CD25) or DPT2 (CD137) (right) shown overlaid. Observed trends are shown in red. DPT color scales were adjusted to emphasize separation of DPT1 and DPT2. Results were obtained using an antibody panel in Supplementary Data 2 and refer to data shown in Fig. 3c.
Supplementary Figure 8 High-dimensional phenotypic diversity calculated on the basis of mass cytometry data decreases with division.
(a) Boxplots showing mean phenotypic diversity of CFSE+ CD8+ T cells within each division state on day 4 (left), day 5 (center), and day 7 (right) of REP (n = 7 biological samples from 2 experiments). Results for day 3 are shown in Fig. 3f. Phenotypic diversity was calculated as an average angular (cosine) distance to an average cell within each division state using arsinh-transformed expression of 18 common surface proteins from antibody panels shown in Supplementary Data 2 and 3 that were not used for gating: CD7, CD25 (IL2Rα), CD27, CD28, CD38, CD45RA, CD45RO, CD57, CD69, CD134 (OX40), CD137 (4-1BB), CD127 (IL7Rα), CD150 (SLAMF1), CD197 (CCR7), CD223 (LAG3), CD272 (BTLA), CD278 (ICOS), and CD279 (PD1). (b) Boxplot showing mean phenotypic diversity on REP days 3, 4, 5, and 7 (n = 28 biological samples containing 4 timepoints from 2 experiments) calculated as an average Euclidean distance to an average cell within each division state using arsinh-transformed expression of 18 common surface proteins shown in (a). (c) Boxplots showing mean phenotypic diversity on REP days 3, 4, 5, and 7 (n = 28 biological samples containing 4 timepoints from 2 experiments) calculated as described in (a) using arsinh-transformed expression of 15 randomly sampled surface proteins in 3 trials (excluded proteins that are shown above each plot). To assess evidence for a decrease in phenotypic diversity with division in (a-c), we used linear mixed-effects models; slope ± standard error (s.e.) and associated P-values are shown above each plot. Boxplots show quartiles with a band at median, whiskers indicating 1.5 IQR, and outliers shown separately.
Supplementary Figure 9 Single-cell RNA-sequencing uncovers the basis of undivided cell state diversity during early divergence in naive CD8+ T-cell expansion.
(a) An experimental outline: 10,000 CFSE+ CD8+ T cells from divisions 0, 1, and 2 were sorted for droplet-based single-cell RNA-sequencing on day 3 of REP. Out of 19,222 genes detected in n = 4,060 cells (division 0: n = 522; division 1: n = 1,777; division 2: n = 1,731; results from 1 experiment), we selected 1,000 most variably expressed genes for subsequent analysis, including a force-directed graph connecting consecutive (-1, 0, +1) division states, diffusion pseudo-time (DPT), individual gene expression, and phenotypic diversity. (b) Fluorescence-activated cell sorting (FACS) gating strategy to sort out CFSE+ CD8+ T cells that divided 0, 1, or 2 times based on CFSE peaks. (c) A force-directed graph constructed using data described in (a) showing division states for each cell. Definition, cell number and percent, division state frequency distribution, and expression of notable genes are shown for 4 regions of interest. (d) Top left: DPT is shown overlaid onto the graph from (c), with colors showing the most likely paths based on diffusion transition probabilities towards two endpoints (DPT1 and DPT2). Color scales were adjusted to emphasize separation of DPT1 and DPT2. Top right: expression of IFNG (encoding IFN-γ), an example of a gene highlighted in (c); see Supplementary Fig. 10 for additional examples. Bottom: expression of IL2RA (encoding CD25) and TNFSRSF9 (encoding CD137) indicate that DPT1 and DPT2 here may be equivalent to those shown in Fig. 3c. (e) A violin plot showing phenotypic diversity of sorted CFSE+ CD8+ T cells from (a). Phenotypic diversity was calculated for each cell as an angular (cosine) distance to an average cell within each division state using expression of 1,000 most variably expressed genes. Violin plots show a kernel density estimate with a boxplot, which contains quartiles with a band at median and whiskers indicating 1.5 IQR. Kruskal-Wallis H test (one-way ANOVA on ranks): P = 1.58e−177; two-sided Wilcoxon Mann-Whitney (WMW) U test comparing division 0 vs. division 1: P = 3.96e−174, comparing division 0 vs. division 2: P = 4.58e−135. WMW P-values were corrected for multiple hypotheses testing using Bonferroni correction. (f) A heatmap showing expression of all genes expressed in ≥10 cells for 50 cells from each region highlighted in (c); cells were selected based on DPT values (DPTlow for regions 1 and 4; DPT1high for region 3; DPT2high for region 2). Genes with relevance to T-cell biology are highlighted below the heatmap, with color indicating expression specific to a given region; black color indicates expression in multiple regions.
Supplementary Figure 10 Selected gene expression projected onto a force-directed graph showing early divergence of naive T cells on day 3 of REP.
A force-directed graph (from Supplementary Fig. 9c) was constructed using expression of 1,000 most variably expressed genes in CFSE+ CD8+ T cells on day 3 of REP that divided 0, 1, or 2 times, with edges connecting cells only in consecutive (-1, 0, +1) divisions. Expression of 23 notable genes is shown projected onto the graph. See Supplementary Fig. 9d for expression of IFNG, IL2RA, and TNFRSF9.
Supplementary Figure 11 Selected single-cell gene expression in regions 1–4 of a force-directed graph showing early divergence of naive T cells on day 3 of REP.
Regions 1-4 were defined in Supplementary Fig. 9c based on cell coordinates in a force-directed graph constructed using expression of 1,000 most variably expressed genes in CFSE+ CD8+ T cells on day 3 of REP. n = 50 cells from each region were then selected based on DPT values: DPTlow for regions 1 and 4, DPT2high for region 2, and DPT1high for region 3. Individual gene expression is shown as a violin plot for genes that are significantly enriched in a given region. Violin plots show a kernel density estimate with a boxplot, which contains quartiles with a band at median and whiskers indicating 1.5 IQR. Kruskal-Wallis H test P < 1e−3 for each gene shown; two-sided WMW U test comparing the highlighted region to each other region in a pairwise manner is noted as ‘*’ for P < 0.05, ‘**’ for P < 0.01, and ‘***’ for P < 0.001. WMW P-values were corrected for multiple hypotheses testing using Bonferroni correction.
Supplementary Figure 12 Single-cell RNA-sequencing on day 3 of expansion shows that high-dimensional phenotypic diversity is higher in undivided T cells.
(a) A violin plot showing phenotypic diversity of sorted CFSE+ CD8+ T cells within each division state on day 3 of REP (n = 522 division 0 cells, n = 1,777 division 1 cells, and n = 1,731 division 2 cells were sequenced in 1 experiment). Phenotypic diversity was calculated for each cell as an angular (cosine) distance to an average cell within each division state using expression of all 19,222 detected genes. Kruskal-Wallis H test (one-way ANOVA on ranks): P = 6.85e−67; two-sided WMW U test comparing division 0 vs. division 1: P = 1.60e−70, comparing division 0 vs. division 2: P = 2.37e−41. (b) Phenotypic diversity was calculated for each cell from (a) as an angular distance to an average cell within each division state using expression of the same genes assessed by mass cytometry in Fig. 3. Kruskal-Wallis H test: P = 1.14e−43; two-sided WMW U test comparing division 0 vs. division 1: P = 8.29e−43, comparing division 0 vs. division 2: P = 5.38e−33. (c) Phenotypic diversity was calculated for each cell from (a) as a Euclidean distance to an average cell within each division state using expression of 1,000 most variably expressed genes. Kruskal-Wallis H test: P = 1.58e−177; two-sided WMW U test comparing division 0 vs. division 1: P = 3.96e−174, comparing division 0 vs. division 2: P = 4.58e−135. WMW P-values in (a-c) were corrected for multiple hypotheses testing using Bonferroni correction. Violin plots show a kernel density estimate with a boxplot, which contains quartiles with a band at median and whiskers indicating 1.5 IQR.
Supplementary Figure 13 T cells expand into new phenotypic niches as they progress through differentiation over 7 d, yet undivided cells continue to have the highest phenotypic diversity.
(a) CFSE+ CD8+ T cells from days 0, 3, 4, 5, and 7 of REP were pooled into a force-directed graph constructed using expression of 27 surface proteins that were not used for gating (Supplementary Data 3), with edges connecting cells only in consecutive (-1, 0, +1) divisions. Day of REP culture (time; left), division state (center), or diffusion pseudo-time (DPT; right) were overlaid onto the graph for all time points. Graph regions were defined using individual marker expression shown in Supplementary Fig. 14. Prominent populations were denoted as T stem cell memory (TSCM)-like cells (CD45RA+ CD45RO- CD27+ CD127+ CCR7+ CTLA4- LAG3- PD1- CD57-) and cells expressing increased levels of dysfunctional state markers (CD27- CTLA4+ LAG3+ PD1+; this includes a smaller subpopulation of cells expressing a senescence marker CD57). DPT was calculated using expression of the same 27 surface proteins and overlaid on the force-directed graph. Observed trends are shown in red. Results were nearly identical when only proteins from the original panel (Supplementary Data 2) were used instead of the extended panel (Supplementary Data 3). (b) Data from (a) shown separately on each day. Results in (a-b) are from 1 donor representative of 5 donors from 3 experiments.
Supplementary Figure 14 Marker expression projected onto a force-directed graph showing naive T-cell expansion on days 0–7.
(a) A force-directed graph (from Supplementary Fig. 13) was constructed using expression of 27 surface proteins (shown in Supplementary Data 3) that were not used for gating on CFSE+ CD8+ T cells from days 0, 3, 4, 5, and 7 of REP, with edges connecting cells only in consecutive (-1, 0, +1) divisions. Expression of 27 surface proteins is shown projected onto the graph. (b) Expression of 2 functional markers of translational activity and mTOR activation (p4EBP1 and pRPS6) is shown projected onto the graph from (a). Results are from 1 donor representative of 5 donors from 3 experiments.
Supplementary Figure 15 Marker expression projected onto a force-directed graph showing naive T-cell differentiation in the presence of vehicle or ibrutinib on days 0–7.
(a-c) Naive CFSE+ T cells were expanded in REP cultures in presence of either vehicle or ibrutinib, collected on days 0, 3, 4, 5, and 7, and assessed by mass cytometry using an antibody panel in Supplementary Data 3. CFSE+ CD8+ T cells were pooled into a force-directed graph constructed using expression of 27 surface proteins that were not used for gating, with edges connecting cells only in consecutive (-1, 0, +1) divisions. (a) Location of vehicle-treated (blue) or ibrutinib-treated (red) cells on the graph. (b) Expression of 27 surface proteins. (c) Expression of 2 functional markers of translational activity and mTOR activation: p4EBP1 and pRPS6. A smaller version of pRPS6 is also shown in Fig. 4c. Division states, time, and DPT for this graph are shown in Fig. 4b. Results are from 1 donor representative of 3 donors from 2 experiments.
Supplementary Figure 16 Functional analysis of TSCM-like and dysfunctional-phenotype T-cell subsets.
(a) Outline of the functional analysis experiments. CFSE+ naive T cells were expanded using REP for 7 days. Next, TSCM-like and dysfunctional-phenotype subsets were sorted out using FACS, re-labeled with CFSE, cultured with new autologous CFSE-negative accessory cells using REP for 3 days, and pulsed with IdU 30 minutes prior to collection and analysis by mass cytometry using an antibody panel in Supplementary Data 4. (b) FACS gating strategy to sort out putative TSCM and dysfunctional subsets based on the observed correlation in single-cell protein expression on day 7 of REP (Supplementary Figs. 13, 14). Putative TSCM cells were defined as single live CD3ε+ CFSE+ antigen-experienced (divided >1 times) CD27+ CD45RA+ PD1- events, and putative dysfunctional T cells were defined as single live CD3ε+ CFSE+ antigen-experienced CD27- PD1+ events. The post-sort purity of each subset was >90%. (c) Contour plots showing mass cytometry analysis of putative TSCM and dysfunctional subsets on day 3 of re-stimulation (gated on live non-apoptotic CFSE+ CD8+ T cells). The functional analysis included the potential to proliferate (CD7 and CD27 expression; the percentage of cells in S phase quantified by IdU incorporation), persist (the percentage of apoptotic cPARP+ cells), and maintain desirable phenotype (lack of expression of dysfunctional markers CTLA4 and LAG3 and of senescence marker CD57). *To measure the percentage of apoptotic (cPARP+) cells, the gating strategy excluded the cPARP- gate (gated on live CFSE+ CD8+ T cells). Results are from 1 experiment.
Supplementary Figure 17 Intracellular cytokine production by TSCM-like and dysfunctional-phenotype T-cell subsets.
Naive CFSE+ T cells were expanded using REP for 7 days. Next, intracellular production of IL-2, IFN-γ, and TNF-α was assessed by stimulating cells with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 4 hours in the presence of a secretion inhibitor. Cells were stained using an antibody panel in Supplementary Data 5 and assessed by mass cytometry. Live non-apoptotic CFSE+ CD8+ T cells were gated as putative TSCM (antigen-experienced CD27+ CD45RA+ CTLA4-) or dysfunctional (antigen-experienced CD27- CTLA4+) cells. Results are from 1 experiment.
Supplementary Figure 18 Single-cell correlation among marker expression and DPT on day 3 of naive T-cell expansion.
Naive CFSE+ T cells were expanded using REP for 3 days and assessed by mass cytometry using an antibody panel in Supplementary Data 3. Spearman’s rank correlation coefficient was calculated among arsinh-transformed expression of 27 surface proteins that were not used for gating, 2 functional markers, and DPT on single live non-apoptotic CFSE+ CD8+ T cells. Results are from 1 donor representative of 5 donors from 3 experiments.
Supplementary Figure 19 Effects of ibrutinib and rapamycin treatment on naive T-cell proliferation, marker expression and DPT over 7 d of REP.
(a-c) Naive CFSE+ T cells were treated with vehicle (DMSO), ibrutinib, or rapamycin for 3, 4, 5, or 7 days in REP culture, assessed by mass cytometry using an antibody panel in Supplementary Data 3, and gated on live non-apoptotic CFSE+ CD8+ T cells. (a) Division state distribution for each day and treatment condition. (b) Heatmaps showing mean log2 fold change over vehicle in arsinh-transformed expression of 27 surface proteins and 2 functional markers for ibrutinib-treated (left) and rapamycin-treated (right) cells for each day and division ID. Observed trends are highlighted with black boxes. (c) Boxplots showing DPT for individual cells separated by treatment, day of culture, and division state. Boxplots show quartiles with a band at median, whiskers indicating 1.5 IQR, and outliers shown separately. DPT was calculated using expression of 27 surface proteins separately for each treatment. All differences were statistically significant (P < 0.001) based on Kruskal-Wallis H test applied to 3 treatment groups, followed by independent two-sided WMW U test applied to each treatment vs. vehicle pair and Bonferroni correction for multiple hypothesis testing. Ibrutinib results are from 1 experiment representative of 3 experiments; rapamycin results are from 1 experiment.
Supplementary Figure 20 Rapamycin halts T-cell differentiation in a naive-like CD127++ state.
(a-c) Vehicle (DMSO) or rapamycin-treated naive CFSE+ T cells were collected from REP cultures on days 3, 4, 5, and 7, assessed by mass cytometry using an antibody panel in Supplementary Data 3, gated on live non-apoptotic CFSE+ CD8+ T cells, and pooled into a force-directed graph constructed using expression of 27 surface proteins, with edges connecting cells only consecutive (-1, 0, +1) divisions. (a) Division state (left), time (center), or DPT (right) are shown overlaid onto the graph. DPT was calculated using expression of the same 27 surface proteins separately for each treatment, and then scaled from 0 to 1 (i.e. DPT values for rapamycin appear inflated on average 2.9x). Graph regions were defined using individual marker expression. Observed trends are shown in red. Location of vehicle-treated (blue) or rapamycin-treated (red) cells is shown on bottom-left. (b) Expression of either 27 surface proteins, and (c) 2 functional markers of translational activity and mTOR activation (p4EBP1 and pRPS6) are shown projected onto the graph from (a). Results are from 1 experiment.
Supplementary Figure 21 Division and time-based characterization of ibrutinib-induced skewing of naive T-cell differentiation toward an TSCM-like state.
(a-c) Naive CFSE+ T cells were treated with vehicle (DMSO) or ibrutinib, collected on days 0, 3, 4, 5, and 7 days of REP culture, assessed by mass cytometry using an antibody panel in Supplementary Data 3, and gated on live non-apoptotic CFSE+ CD8+ T cells. (a) Contour plots showing distribution of angular distances to an average TSCM-like (TSCM) or dysfunctional-phenotype (Dysf.) cell for all vehicle and ibrutinib-treated cells from Fig. 4b on each day of REP culture. Angular distances were calculated based on the surface proteins used to define TSCM-like and dysfunctional-phenotype gates. (b) Boxplots of single-cell data from (a) on days 3 and 7 separated by division state. Boxplots show quartiles with a band at median and whiskers indicating 1.5 IQR; outliers are not shown. P-values were calculated using independent two-sided WMW U test. *P < 0.05, **P < 0.01, ***P < 0.001. (c) Contour plots showing arsinh-transformed protein expression on reference naive, TSCM-like, and dysfunctional-phenotype subsets used for distance calculation in (a-b). Results are from 1 experiment representative of 3 experiments.
Supplementary Figure 22 Delaying ibrutinib treatment until day 3 reduces skewing of naive T-cell differentiation toward an TSCM-like state.
Naive CFSE+ T cells were treated with (1) vehicle (DMSO) on days 0-7, (2) ibrutinib on days 0-7, or (3) ibrutinib on days 3-7 only (and vehicle prior). Cells were collected on day 7 of REP culture, assessed by mass cytometry using an antibody panel in Supplementary Data 4, and gated on live non-apoptotic CFSE+ CD8+ T cells. Contour plots show expression of T-cell differentiation markers (CD45RA and CD45RO), TSCM markers (CD27 and CD11a), and dysfunctional markers (CTLA4 and LAG3). Results are from 1 experiment.
Supplementary Figure 23 Ibrutinib effect on bulk T-cell intracellular cytokine production and apoptosis.
Naive CFSE+ T cells were treated with (1) vehicle (DMSO) on days 0-7, (2) ibrutinib on days 0-7, or (3) ibrutinib until day 3 only (and vehicle starting on day 3). Next, intracellular production of IL-2, IFN-γ, and TNF-α was assessed by stimulating cells with PMA and ionomycin for 4 hours in the presence of a secretion inhibitor on either (a) day 7 of REP culture, or (b) after 1 day of rest after day 7 (without anti-CD3ε antibody, IL-2, and small molecule treatment). Cells were stained using an antibody panel in Supplementary Data 5, examined by mass cytometry, gated on live CFSE+ CD8+ T cells to assess percent of apoptotic (cPARP+) cells, and then gated on non-apoptotic cells to assess intracellular cytokine production. Results are from 1 experiment.
Supplementary Figure 24 Effect of ibrutinib on intracellular production of IFN-γ and TNF-α by effector T cells.
Naive CFSE+ T cells were treated with (1) vehicle (DMSO) on days 0-7, (2) ibrutinib on days 0-7, or (3) ibrutinib until day 3 only (and vehicle starting on day 3). Next, intracellular cytokine production was assessed by stimulating cells with PMA and ionomycin for 4 hours in the presence of a secretion inhibitor on either (a) day 7 of REP culture, or (b) after 1 day of rest after day 7 (without anti-CD3ε antibody, IL-2, and small molecule treatment). Cells were stained using an antibody panel in Supplementary Data 5, examined by mass cytometry, gated on live non-apoptotic CFSE+ CD8+ effector (CCR7-) T cells to assess intracellular IFN-γ and TNF-α production. Results are from 1 experiment.
Supplementary Figure 25 Effect of early ibrutinib treatment on skewing of naive T-cell differentiation toward a TSCM-like state.
Naive CFSE+ T cells were treated with (1) vehicle (DMSO) on days 0-7, (2) ibrutinib on days 0-7, or (3) ibrutinib until day 3 only (and vehicle starting on day 3). On day 7 of REP culture, intracellular cytokine production was assessed by stimulating cells with PMA and ionomycin for 4 hours. Cells were analyzed by mass cytometry using an antibody panel in Supplementary Data 5, and gated on live non-apoptotic CFSE+ CD8+ T cells. Contour plots show expression of a TSCM marker (CD27) and dysfunctional markers (CTLA4 and LAG3) with and without cell stimulation. Results are from 1 experiment.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–25
Supplementary Data 1
Development panel
Supplementary Data 2
Main naive T-cell differentiation panel
Supplementary Data 3
Extended naive T-cell differentiation panel
Supplementary Data 4
Adjusted naive T-cell differentiation panel
Supplementary Data 5
Cytokine panel
Supplementary Data 6
T-cell marker annotation
Supplementary Data 7
Relationship between T-cell markers and DPT
Supplementary Data 8
Effects of small molecule inhibitors on total cell counts and cell loss
Rights and permissions
About this article
Cite this article
Good, Z., Borges, L., Vivanco Gonzalez, N. et al. Proliferation tracing with single-cell mass cytometry optimizes generation of stem cell memory-like T cells. Nat Biotechnol 37, 259–266 (2019). https://doi.org/10.1038/s41587-019-0033-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0033-2
This article is cited by
-
Identification, sorting and profiling of functional killer cells via the capture of fluorescent target-cell lysate
Nature Biomedical Engineering (2023)
-
CAR immune cells: design principles, resistance and the next generation
Nature (2023)
-
Advanced technologies for single-cell in situ protein profiling
Science China Chemistry (2022)
-
Extended live-cell barcoding approach for multiplexed mass cytometry
Scientific Reports (2021)
-
Single-cell lipidomics with high structural specificity by mass spectrometry
Nature Communications (2021)