Abstract
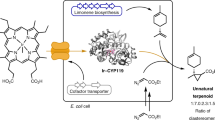
Biosynthesis is an environmentally benign and renewable approach that can be used to produce a broad range of natural and, in some cases, new-to-nature products. However, biology lacks many of the reactions that are available to synthetic chemists, resulting in a narrower scope of accessible products when using biosynthesis rather than synthetic chemistry. A prime example of such chemistry is carbene-transfer reactions1. Although it was recently shown that carbene-transfer reactions can be performed in a cell and used for biosynthesis2,3, carbene donors and unnatural cofactors needed to be added exogenously and transported into cells to effect the desired reactions, precluding cost-effective scale-up of the biosynthesis process with these reactions. Here we report the access to a diazo ester carbene precursor by cellular metabolism and a microbial platform for introducing unnatural carbene-transfer reactions into biosynthesis. The α-diazoester azaserine was produced by expressing a biosynthetic gene cluster in Streptomyces albus. The intracellularly produced azaserine was used as a carbene donor to cyclopropanate another intracellularly produced molecule—styrene. The reaction was catalysed by engineered P450 mutants containing a native cofactor with excellent diastereoselectivity and a moderate yield. Our study establishes a scalable, microbial platform for conducting intracellular abiological carbene-transfer reactions to functionalize a range of natural and new-to-nature products and expands the scope of organic products that can be produced by cellular metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The DNA sequences of plasmids used in this study have been deposited in the public version of the JBEI registry (http://public-registry.jbei.org). Accession codes are provided in Supplementary Table 2 (Part ID). The sequences and annotation of the aza BGC is available at GenBank (NZ_BEVZ01000003.1) spanning bases 82400–111549. The determined structure has been deposited in the PDB (8FBC). The MS proteomics data have been deposited at the ProteomeXchange Consortium through the PRIDE63 partner repository under the dataset identifier PXD037509. Source data are provided with this paper.
Change history
21 December 2023
In the version of the article originally published, the panel label ‘d’ was missing in Fig. 4. The error has been corrected in the HTML and PDF versions of the article.
References
Arnold, F. H. Directed evolution: bringing new chemistry to life. Angew. Chem. Int. Ed. Engl. 57, 4143–4148 (2018).
Wallace, S. & Balskus, E. P. Interfacing microbial styrene production with a biocompatible cyclopropanation reaction. Angew. Chem. Int. Ed. Engl. 54, 7106–7109 (2015).
Huang, J. et al. Unnatural biosynthesis by an engineered microorganism with heterologously expressed natural enzymes and an artificial metalloenzyme. Nat. Chem. 13, 1186–1191 (2021).
Davies, H. M. L. & Manning, J. R. Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Doyle, M. P., Duffy, R., Ratnikov, M. & Zhou, L. Catalytic carbene insertion into C-H bonds. Chem. Rev. 110, 704–724 (2010).
Ford, A. et al. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 115, 9981–10080 (2015).
Zhu, D., Chen, L. F., Fan, H. L., Yao, Q. L. & Zhu, S. F. Recent progress on donor and donor-donor carbenes. Chem. Soc. Rev. 49, 908–950 (2020).
Kluger, R. Thiamin diphosphate: a mechanistic update on enzymic and nonenzymic catalysis of decarboxylation. Chem. Rev. 87, 863–876 (1987).
Lee, J. K. & Houk, K. N. A proficient enzyme revisited: the predicted mechanism for orotidine monophosphate decarboxylase. Science 276, 942–945 (1997).
Meyer, D., Neumann, P., Ficner, R. & Tittmann, K. Observation of a stable carbene at the active site of a thiamin enzyme. Nat. Chem. Biol. 9, 488–490 (2013).
Cochrane, A. R. et al. The natural product lepidiline A as an N-heterocyclic carbene ligand precursor in complexes of the type [Ir(cod)(NHC)PPh3)]X: synthesis, characterisation, and application in hydrogen isotope exchange catalysis. Catalysts 10, 161 (2020).
Cheng, R. H. et al. Implications for an imidazole-2-yl carbene intermediate in the rhodanase-catalyzed C-S bond formation reaction of anaerobic ergothioneine biosynthesis. ACS Catal. 11, 3319–3334 (2021).
Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339, 307–310 (2013).
Brandenberg, O. F., Fasan, R. & Arnold, F. H. Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr. Opin. Biotechnol. 47, 102–111 (2017).
Liu, Z. & Arnold, F. H. New-to-nature chemistry from old protein machinery: carbene and nitrene transferases. Curr. Opin. Biotechnol. 69, 43–51 (2021).
Wallace, S. & Balskus, E. P. Designer micelles accelerate flux through engineered metabolism in E. coli and support biocompatible chemistry. Angew. Chem. Int. Ed. Engl. 55, 6023–6027 (2016).
Wong, H. N. C. et al. Use of cyclopropanes and their derivatives in organic synthesis. Chem. Rev. 89, 165–198 (1989).
Talele, T. T. The “cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 59, 8712–8756 (2016).
Fulton, J. R., Aggarwal, V. K. & de Vicente, J. The use of tosylhydrazone salts as a safe alternative for handling diazo compounds and their applications in organic synthesis. Eur. J. Org. Chem. 2005, 1479–1492 (2005).
Morandi, B. & Carreira, E. M. Iron-catalyzed cyclopropanation in 6 M KOH with in situ generation of diazomethane. Science 335, 1471–1474 (2012).
Zhang, L. M., DeMuynck, B. M., Paneque, A. N., Rutherford, J. E. & Nagib, D. A. Carbene reactivity from alkyl and aryl aldehydes. Science 377, 649–654 (2022).
Xia, Y., Qiu, D. & Wang, J. B. Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem. Rev. 117, 13810–13889 (2017).
Damiano, C., Sonzini, P. & Gallo, E. Iron catalysts with N-ligands for carbene transfer of diazo reagents. Chem. Soc. Rev. 49, 4867–4905 (2020).
Nawrat, C. C. & Moody, C. J. Natural products containing a diazo group. Nat. Prod. Rep. 28, 1426–1444 (2011).
Kawai, S. et al. Complete biosynthetic pathway of alazopeptin, a tripeptide consisting of two molecules of 6-diazo-5-oxo-l-norleucine and one molecule of alanine. Angew. Chem. Int. Ed. 60, 10319–10325 (2021).
Le Maux, P., Nicolas, I., Chevance, S. & Simonneaux, G. Chemical reactivity of 6-diazo-5-oxo-l-norleucine (DON) catalyzed by metalloporphyrins (Fe,Ru). Tetrahedron 66, 4462–4468 (2010).
Sugai, Y., Katsuyama, Y. & Ohnishi, Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat. Chem. Biol. 12, 73–75 (2016).
Ma, G. L. et al. Biosynthesis of tasikamides via pathway coupling and diazonium-mediated hydrazone formation. J. Am. Chem. Soc. 144, 1622–1633 (2022).
Bartz, Q. R. et al. Isolation and characterization of azaserine. Nature 173, 72–73 (1954).
Stock, C. C., Clarke, D. A., Reilly, H. C., Rhoads, C. P. & Buckley, S. M. Azaserine, a new tumour-inhibitory substance: studies with crocker mouse sarcoma 180. Nature 173, 71–72 (1954).
Key, H. M. et al. Beyond iron: iridium-containing P450 enzymes for selective cyclopropanations of structurally diverse alkenes. ACS Cent. Sci. 3, 302–308 (2017).
Lee, M. D. et al. New antitumor antibiotic, LL-D05139β. Fermentation, isolation, structure determination and biological activities. J. Antibiot. 40, 1657–1663 (1987).
Matsuda, K. et al. Genome mining of amino group carrier protein-mediated machinery: discovery and biosynthetic characterization of a natural product with unique hydrazone unit. ACS Chem. Biol. 12, 124–131 (2017).
Twigg, F. F. et al. Identifying the biosynthetic gene cluster for triacsins with an N-hydroxytriazene moiety. ChemBioChem 20, 1145–1149 (2019).
Williams, M. V. & Tritz, G. J. Studies on the modes of action of azaserine inhibition of Escherichia coli. Potentiation of phenylalanine reversal. J. Antimicrob. Chemother. 3, 65–77 (1977).
Geisen, S. M. et al. Direct alkylation of deoxyguanosine by azaserine leads to O6-carboxymethyldeoxyguanosine. Chem. Res. Toxicol. 34, 1518–1529 (2021).
Gober, J. G. et al. P450-mediated non-natural cyclopropanation of dehydroalanine-containing thiopeptides. ACS Chem. Biol. 12, 1726–1731 (2017).
Zhang, R. J. K. et al. Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization. Nature 565, 67–72 (2019).
Nguyen, K. T. et al. Characterization of a thermophilic cytochrome P450 of the CYP203A subfamily from Binh Chau hot spring in Vietnam. FEBS Open Bio 11, 124–132 (2021).
Ost, T. W. B. et al. Oxygen activation and electron transfer in flavocytochrome P450BM3. J. Am. Chem. Soc. 125, 15010–15020 (2003).
McKenna, R. & Nielsen, D. R. Styrene biosynthesis from glucose by engineered E. coli. Metab. Eng. 13, 544–554 (2011).
McKenna, R., Thompson, B., Pugh, S. & Nielsen, D. R. Rational and combinatorial approaches to engineering styrene production by Saccharomyces cerevisiae. Microb. Cell Fact. 13, 123 (2014).
Lee, K., Bang, H. B., Lee, Y. H. & Jeong, K. J. Enhanced production of styrene by engineered Escherichia coli and in situ product recovery (ISPR) with an organic solvent. Microb. Cell Fact. 18, 79 (2019).
Lin, F. M., Ferguson, K. L., Boyer, D. R., Lin, X. X. N. N. & Marsh, E. N. G. Isofunctional enzymes PAD1 and UbiX catalyze formation of a novel cofactor required by ferulic acid decarboxylase and 4-hydroxy-3-polyprenylbenzoic acid decarboxylase. ACS Chem. Biol. 10, 1137–1144 (2015).
Brandenberg, O. F., Chen, K. & Arnold, F. H. Directed evolution of a cytochrome P450 carbene transferase for selective functionalization of cyclic compounds. J. Am. Chem. Soc. 141, 8989–8995 (2019).
Fischer, M. & Sawers, R. G. A universally applicable and rapid method for measuring the growth of streptomyces and other filamentous microorganisms by methylene blue adsorption-desorption. Appl. Environ. Microbiol. 79, 4499–4502 (2013).
Aziz, R. K. et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 9, 75 (2008).
Navarro-Munoz, J. C. et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 16, 60–68 (2020).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Winter, G., Lobley, C. M. C. & Prince, S. M. Decision making in xia2. Acta Crystallogr. D 69, 1260–1273 (2013).
Mccoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Lee, T. S. et al. BglBrick vectors and datasheets: a synthetic biology platform for gene expression. J. Biol. Eng. 5, 12 (2011).
Yuzawa, S. et al. Short-chain ketone production by engineered polyketide synthases in Streptomyces albus. Nat. Commun. 9, 4569 (2018).
Ehrlich, J. et al. Process for producing azaserine. US patent 2996435A (1961).
Chen, Y., Gin, J. & Petzold, C. Discovery proteomic (DIA) LC-MS/MS data acquisition and analysis V.2. Protocols.io https://doi.org/10.17504/protocols.io.e6nvwk1z7vmk/v2 (2022).
Demichev, V., Messner, C. B., Vernardis, S. I., Lilley, K. S. & Ralser, M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 17, 41–44 (2020).
Ahrne, E., Molzahn, L., Glatter, T. & Schmidt, A. Critical assessment of proteome-wide label-free absolute abundance estimation strategies. Proteomics 13, 2567–2578 (2013).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Madeira, F. et al. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 50, W276–W279 (2022).
Acknowledgements
This work was supported by Joint BioEnergy Institute (https://www.jbei.org), which is supported by the DOE, Office of Science, Office of Biological and Environmental Research under contract DE-AC02-05CH11231 and National Science Foundation grant 2027943. We thank H. Celik and the staff at UC Berkeley’s NMR facility in the College of Chemistry (CoC-NMR) for spectroscopy assistance. Instruments in the CoC-NMR are supported in part by NIH S10OD024998.
Author information
Authors and Affiliations
Contributions
J.H. and J.D.K. conceived the project. J.H. and J.D.K. wrote the manuscript with input from all of the other authors. J.D.K., A.M., J.F.H. and D.S.C. provided project guidance. A.Q. and K.D. synthesized the chemical standards. J.H., P.C.-M. and D.V.C. identified the gene cluster. J.H.P. determined the crystal structure. Q.D. purified the proteins. J.H., R.K. and E.E.K.B. performed MS. Y.C. performed proteomics analysis. J.H. conducted the other experiments. E.P.B., P.D.A., T.R.N. and C.J.P. provided resources. All of the authors contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.D.K. has a financial interest in Amyris, Demetrix, Maple Bio, Lygos, Napigen, Berkeley Yeast, Zero Acre Farms, Ansa Biotechnologies, Apertor Pharmaceuticals, ResVit Bio and Cyklos Materials. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Frances Arnold, Nicholas Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Effect of Na2S2O4 on the activity of haemin and Ir(Me)MPIX for the reaction of styrene with azaserine.
a, Addition of Na2S2O4 decreased the reaction yield when using Ir(Me)MPIX as catalyst. TON, turn-over number. Reaction conditions are described in Fig. 2 legend. Data are mean value for 2 reaction replicates. b, Na2S2O4 is necessary for the activity of haemin toward the reaction. EIC ([M+H]+, m/z 250.1074) for target products. The traces are representative of two reaction replicates. The reaction contained 5 mM styrene, 5 mM azaserine, 10 µM haemin or no catalyst, 0 or 10 mM Na2S2O4, 5 vol% ethanol, and M9-N buffer and was conducted at 22 °C under aerobic conditions for 18 h. Standard, chemically synthesized authentic standard mixture of the four diastereomers.
Extended Data Fig. 2 Azaserine toxicity on E. coli and S. albus.
a, Azaserine is toxic to E. coli BL21(DE3). b, Azaserine does not affect the growth of S. albus under tested concentrations. Biomass was normalized to that of culture without addition of azaserine. Data are mean ± s.d.; n = 3 biological replicates.
Extended Data Fig. 3 Expression of azaserine gene cluster from S. fragilis in S. albus.
a, Bioinformatic annotation of the azaserine gene cluster and comparison with the biosynthetic gene clusters for some natural N–N bond-containing compounds. b, Proteomic analysis of the azaserine gene cluster when expressed in S. albus. Data are mean ± s.d.; n = 3 biological replicates.
Extended Data Fig. 4 Differences in azaserine degradation in various media.
a, Azaserine titre continues to decrease after removal of S. albus cells. The azaserine-producing S. albus was grown in TSB medium. After 24 h, the cells were removed from culture broth using a 0.22-µm sterile filter, the filtrate was incubated at 30 °C (labelled as 0 h), and the azaserine concentration was monitored at different time points. b, Azaserine is stable in fresh TSB medium of normal pH 7.3 or adjusted pH 8.4. Equal volume of azaserine stock was added to a final concentration of about 35 mg/L at 0 h. Data are mean ± s.d.; n = 3 biological (a) or technical (b) replicates.
Extended Data Fig. 5 Purified P450-T2 WT and mutant catalysing the reaction of styrene with azaserine in vitro.
a, Coomassie Blue stained SDS-PAGE gel of purified protein P450-T2 WT (left) and P450-T2-5 mutant (right). Each lane is a sample from fractions collected during ion exchange purification. b, Purified P450-T2 WT and P450-T2-5 mutant proteins catalyse the reaction in vitro with high diastereoselectivity. 5th, P450-T2-5 mutant. Reaction conditions: 5 mM styrene, 5 mM azaserine, 10 µM enzyme, 10 mM Na2S2O4, 5 vol% ethanol, M9-N buffer, conducted at 22 °C under aerobic condition for 18 h. Ptotal, sum area for all diastereomers. Grey bars indicate the dr. Data are mean ± s.d.; n = 3 reaction replicates.
Extended Data Fig. 6 CYP203A1 WT and axial ligand mutants for the reaction of styrene with azaserine.
EIC ([M+H]+, m/z 250.1074) for target products. Representative traces for two repeated experiments. The reactions contained 5 mM styrene, 5 mM azaserine, E. coli cells with concentration of 30 OD600 as catalyst, 5 vol% ethanol, and M9-N buffer and were conducted at 22 °C under aerobic conditions for 18 h.
Extended Data Fig. 7 Conserved amino acids selected for saturation mutagenesis.
The residue labelled in blue is the haem ligand cysteine in those P450s. Residues labelled in red are two conserved residues previously reported to affect the catalytic behaviour in P450 BM3. Sequences were aligned with Clustal Omega64.
Extended Data Fig. 8 Exploration of the conditions for styrene biosynthesis in S. albus.
a, Heterologously expressing PAL2 and FDC1 are sufficient to generate styrene. pAZA121 and pAZA138 are two integration plasmids used to introduce the styrene pathway into S. albus. b, Production of styrene by engineered S. albus grown in TSB medium supplemented with additional 0 mM, 2 mM or 4 mM Phe. Data are mean ± s.d.; n = 3 biological samples.
Extended Data Fig. 9 Characterization and time course of accumulation of the final products.
a, MS/MS (20 eV) spectra of the P1 standard (red) and the biosynthesized major product (black). b, Biosynthesis of final products during the 96-h fermentation process. The 24-h data are not presented because small quantities of product were observed (<10 µg/L), and thus the titre could not be accurately calculated. 1B medium with 4 mM Phe was used to generate the final products. Data are mean ± s.d.; n = 3 biological samples.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 (DNA and proteins sequences, synthesis of compounds), Supplementary Figs. 1–38 and Supplementary References.
Rights and permissions
About this article
Cite this article
Huang, J., Quest, A., Cruz-Morales, P. et al. Complete integration of carbene-transfer chemistry into biosynthesis. Nature 617, 403–408 (2023). https://doi.org/10.1038/s41586-023-06027-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06027-2
This article is cited by
-
Ultrafast photoinduced C-H bond formation from two small inorganic molecules
Nature Communications (2024)
-
Carbene chemistry for unnatural biosynthesis
Science China Life Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.