Abstract
Oncogene amplification on extrachromosomal DNA (ecDNA) drives the evolution of tumours and their resistance to treatment, and is associated with poor outcomes for patients with cancer1,2,3,4,5,6. At present, it is unclear whether ecDNA is a later manifestation of genomic instability, or whether it can be an early event in the transition from dysplasia to cancer. Here, to better understand the development of ecDNA, we analysed whole-genome sequencing (WGS) data from patients with oesophageal adenocarcinoma (EAC) or Barrett’s oesophagus. These data included 206 biopsies in Barrett’s oesophagus surveillance and EAC cohorts from Cambridge University. We also analysed WGS and histology data from biopsies that were collected across multiple regions at 2 time points from 80 patients in a case–control study at the Fred Hutchinson Cancer Center. In the Cambridge cohorts, the frequency of ecDNA increased between Barrett’s-oesophagus-associated early-stage (24%) and late-stage (43%) EAC, suggesting that ecDNA is formed during cancer progression. In the cohort from the Fred Hutchinson Cancer Center, 33% of patients who developed EAC had at least one oesophageal biopsy with ecDNA before or at the diagnosis of EAC. In biopsies that were collected before cancer diagnosis, higher levels of ecDNA were present in samples from patients who later developed EAC than in samples from those who did not. We found that ecDNAs contained diverse collections of oncogenes and immunomodulatory genes. Furthermore, ecDNAs showed increases in copy number and structural complexity at more advanced stages of disease. Our findings show that ecDNA can develop early in the transition from high-grade dysplasia to cancer, and that ecDNAs progressively form and evolve under positive selection.
Similar content being viewed by others
Main
EAC is a highly lethal cancer that can arise from Barrett’s oesophagus, a relatively common, pre-cancerous metaplastic condition that affects around 1.6% of the US population7. In addition to epidemiological and clinical features such as chronic gastro-oesophageal reflux disease, the age of the patient and the size of the Barrett’s oesophagus lesion8,9, changes in genomic copy number within Barrett’s oesophagus lesions have been implicated in the development of EAC7,10,11,12,13,14,15. These changes include oncogene amplification, which frequently occurs on circular ecDNA particles (ecDNAs)3. ecDNAs are found in some of the most aggressive forms of cancer—including EAC, the highly accessible chromatin and altered cis- and trans-gene regulation of which enhance oncogenic transcriptional programs16,17,18,19,20. ecDNAs lack centromeres, and are consequently subject to random inheritance during cell division, driving intratumoral genetic heterogeneity6. These unique features of ecDNAs contribute to aggressive tumour growth, accelerated evolution and resistance to treatment. Patients with ecDNA-containing tumours have significantly shorter survival, even compared to other forms of genomic focal amplification3. Computational tools can detect ecDNA in WGS data from biopsies21,22,23. However, the relative paucity of pre-cancer-to-cancer longitudinal studies, together with the challenges of interpreting clonality in the face of non-Mendelian genetics, have made it difficult to determine whether ecDNAs arise early in tumorigenesis and contribute to the transformation of dysplasia into cancer. Previous reports have hypothesized or reported the existence of ecDNA in pre-cancer samples derived from individuals with Barrett’s oesophagus11,14,24,25 suggesting that ecDNA has a role in the malignant transformation to EAC11. Two surveillance studies of patients with Barrett’s oesophagus, including a longitudinal case–control study with multi-regional WGS sampling, and a completely independent, cross-sectional surveillance cohort, with full histological correlatives, provided us with an opportunity to study the role of ecDNA in the transition from Barrett’s oesophagus to EAC.
Study samples
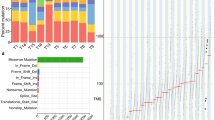
We analysed WGS data from a Cambridge University cross-sectional surveillance cohort of 206 patients with biopsy-validated Barrett’s oesophagus (Supplementary Table 1). This Cambridge cohort included 42 patients with non-dysplastic Barrett’s oesophagus or low-grade dysplasia (LGD) who never developed high-grade dysplasia (HGD) or EAC during follow-up; 25 patients with HGD; 51 patients with early-stage (stage I) EAC; and 88 patients with late-stage (stage II–IV) EAC (Fig. 1a) using the American Joint Committee on Cancer (AJCC) staging system26. Histology and WGS sequencing were performed on the same biopsies (Methods, ‘Cambridge sample selection’). We also analysed 20 EAC tumours from The Cancer Genome Atlas (TCGA) oesophageal carcinoma study27 (ESCA), composed of 6 early-stage and 14 late-stage tumours.
a, Breakdown of the histological disease states among patients with Barrett’s oesophagus in the Cambridge selected cross-sectional study, representing the highest disease state for that patient. NDBE, non-dysplastic Barrett’s oesophagus. b, The FHCC cohort consisted of 80 patients for whom biopsies were collected prospectively. The cohort was separated later into two groups of 40 patients who had cancer outcomes (CO) and non-cancer outcomes (NCO). c, Sample collection at time points TP-1 and TP-2 for sequencing biopsies and histology biopsies. Two sequencing biopsies were collected at each time point. Before sequencing, ethylenediaminetetraacetic acid (EDTA) application and microdissection were performed to isolate Barrett’s oesophagus (BE) tissue and improve purity for sequencing. Highlighted box indicates isolated Barrett’s oesophagus tissue (box width indicates approximately 50 µm). d, WGS biopsies and histology biopsies were collected independently. Some histology and sequencing biopsies were taken at the same level of the oesophagus (on-level), and some histology biopsies fell within a ±1-cm window of the measured height of the sequencing biopsy (windowed histology). e, Experimental workflow for analysing the WGS samples. A brief overview of the process by which biopsies were selected, sequenced and characterized by AmpliconArchitect.
We analysed WGS data from oesophageal biopsies collected in an independent, prospectively collected case–control study conducted at the Fred Hutchinson Cancer Center (FHCC) of patients with Barrett’s oesophagus14 (Fig. 1b), including 40 patients with a cancer outcome and 40 patients who did not develop cancer (non-cancer outcome) during the study period or during follow-up (mean: 10.5 years; Supplementary Table 2). At least two biopsies for WGS were obtained by isolating epithelial tissue (Fig. 1c) from the Barrett’s oesophagus at each of the two primary study time points—time point 1 (TP-1) and time point 2 (TP-2)—which were, on average, 2.9 years and 3.4 years apart for patients with a cancer outcome and those with a non-cancer outcome, respectively (Supplementary Table 1). Histology samples were also collected independently of the sequencing biopsies, including from the same, or close to the same, level in the oesophagus (Fig. 1d). At TP-2, biopsies from the same level of the oesophagus (or as close as possible) as the EAC were used for sequencing (Methods, ‘FHCC cohort histology’). For the resected tumour from patient 391, sequencing was also available. We applied the AmpliconArchitect method for ecDNA detection (Fig. 1e and Methods, ‘ecDNA detection and characterization’), after identifying seed regions of possible focal amplifications in copy number calls. The resulting genome graphs described the fine structure of the amplicon, and explorations of those graphs were subsequently classified by AmpliconClassifier as specific amplicon types (Supplementary Table 3 and Supplementary Information).
ecDNA and Barrett’s oesophagus
ecDNA was not detected in any of the non-dysplastic Barrett’s oesophagus samples or any of the LGD samples in the cross-sectional Barrett’s oesophagus surveillance Cambridge cohort (Extended Data Fig. 1a). By contrast, ecDNA was found in tumours from 13 out of 51 patients (25%) with early-stage (stage I) EAC and in tumours from 38 out of 88 patients (43%) with late-stage tumours (stage II–IV) (Fig. 2a). The occurrence of ecDNA was significantly enriched in early-stage EAC versus non-dysplastic Barrett’s oesophagus or LGD (Fig. 2b; Fisher’s exact test, P = 1.8 × 10−4, one-sided) with an increased frequency of ecDNA in late-stage compared to early-stage tumours (Extended Data Fig. 1b; odds ratio = 2.2, confidence interval = 1.0–4.7, Fisher’s exact test, P = 0.027, one-sided). ecDNA was detected in a nearly identical fraction of an independent cohort of late-stage EAC tumours from TCGA (6 out of 14 tumours; 43%).
a, Characterization of the ecDNA status and cancer stage of patient samples from the Cambridge cohorts of patients with early- and late-stage EAC. b, Comparison of the ecDNA status and histological group of samples reveals an association between ecDNA and early-stage EAC. The odds ratio (OR) and the confidence interval (CI) of the OR are shown. c, Characterization of the ecDNA status and on-level histology of samples collected for FHCC CO patients across time points TP-1 and TP-2 for the two oesophageal sequencing samples (‘upper’ and ‘lower’). The maximum histology of any biopsy from that time point is also shown. Asterisk indicates cancer diagnosis made at next endoscopy (1.44 and 8.16 months after TP-2 for patients 568 and 772, respectively). d, Comparison of ecDNA status in any FHCC patient sample and cancer-outcome status among patients reveals an association between ecDNA and cancer outcome. e, Among FHCC CO patients, the proportion of TP-1 samples without HGD or EAC in on-level histology (having Barrett’s oesophagus or LGD) versus with HGD in the on-level histology, separated by ecDNA status, shows an enrichment for ecDNA with advanced disease status (Fisher’s exact test, one-sided). f, Among FHCC CO patients, the proportion of TP-2 samples without EAC in on-level histology (having HGD or Barrett’s oesophagus) versus with EAC in on-level histology, separated by ecDNA status, shows an association between ecDNA and the development of EAC (Fisher’s exact test, one-sided).
The FHCC study incorporated multi-regional, longitudinal sampling from before and at cancer diagnosis. We examined the development of ecDNA over time in biopsies from patients with Barrett’s oesophagus tissue that progressed to an EAC end-point versus those patients with Barrett’s oesophagus tissue that remained benign at the highest detectable disease state. At cancer diagnosis (TP-2), ecDNA was detected in samples from 11 out of 40 patients with a cancer outcome who developed EAC (28%) (Fig. 2c and Extended Data Fig. 2), consistent with the 25% frequency of ecDNA that was found in the Cambridge cohort of patients with early-stage cancer. ecDNA was detected in biopsies from only one out of 40 patients with a non-cancer outcome (Extended Data Fig. 3). Notably for the non-cancer-outcome ecDNA biopsies, KRAS was amplified (Extended Data Fig. 4); however, the patient died of causes unrelated to Barrett’s oesophagus 2.84 years after TP-2.
We also analysed 20 long-term follow-up samples collected from 10 patients with a non-cancer outcome (median 9.6 years after TP-2) with Barrett’s oesophagus tissue that maintained non-dysplastic Barrett’s oesophagus or LGD status and remained ecDNA-negative (Extended Data Fig. 5a). The median duration of follow-up was 10.5 years for the FHCC patients with a non-cancer outcome, with 85% being followed for more than 5 years (Supplementary Table 2 and Extended Data Fig. 5b). Furthermore, in both time points together, ecDNA was found in samples from one out of 40 patients with a non-cancer outcome and in samples from 13 out of 40 patients with a cancer outcome (Fig. 2d), showing a highly significant association between ecDNA in Barrett’s oesophagus biopsies and progression to EAC (Fisher’s exact test, P = 3.3 × 10−4, one-sided).
ecDNA can arise in high-grade dysplasia
The design of the longitudinal case–control FHCC study enabled the timing of ecDNA development in Barrett’s oesophagus segments to be determined in patients with a cancer outcome. Notably, ecDNA was found at TP-1, before the development of cancer, in biopsy tissues from 7 out of 40 patients (18%), and all 7 of these individuals subsequently developed EAC (Fig. 2d). In addition, at TP-1, HGD was detected in at least one histology biopsy for 27 out of 40 patients (67.5%). Six TP-1 samples that contained ecDNA could be matched to an on-level histology biopsy, all showing HGD (Fig. 2e). By contrast, 46% (21 out of 46) of the ecDNA-negative TP-1 sequencing biopsies could be matched to on-level HGD, indicating a significant association of ecDNA and HGD in the pre-cancer samples (Fig. 2e; Fisher’s exact test, P = 0.015, one-sided).
In samples from patients with a cancer outcome that were collected at TP-2, when cancer was first diagnosed, we associated 54 sequencing biopsies to on-level histology. ecDNAs were identified in 11 of these sequencing biopsies, 9 of which (82%) were associated with on-level EAC, with the remaining 2 being associated with on-level HGD (Fig. 2f). By contrast, among the remaining 43 ecDNA-negative biopsies, only 20 out of 43 (47%) were associated with on-level EAC, with the remaining 23 (53%) being associated with on-level Barrett’s oesophagus or HGD (Fig. 2f; Fisher’s exact test, P = 0.037, one-sided). The specificity of the association of ecDNA with a worsened pathological status at both time points suggests that ecDNAs are enriched in Barrett’s oesophagus clones that become cancer. In the Cambridge cohort, ecDNA was detected in only one of the 25 patients with HGD (Extended Data Fig. 1a). However, in that cohort, HGD was treated immediately after detection, so it was not possible to determine whether the HGD samples would subsequently have progressed to cancer.
TP53 alterations and ecDNA formation
We analysed a number of properties related to the samples in the context of ecDNA, ranging from purity and ploidy (Supplementary Table 4 and Supplementary Fig. 4) to other genomic features, such as TP53. Disruption of TP53 enables genomic instability13,14,28,29, and we found a strong association in both FHCC and Cambridge samples between the TP53 alteration status (Methods, ‘TP53 alteration analysis’) and the presence of ecDNA (Extended Data Fig. 6a,b). All eight FHCC samples in which ecDNA was found before cancer diagnosis (TP-1) showed biallelic disruption of TP53. The appearance of ecDNA as a subset of TP53-altered cases suggests that the prior loss of TP53 enables ecDNA formation.
Whole-genome duplication (WGD) and chromothripsis are tied closely to genome instability11,30,31,32,33, and those mechanisms might contribute to ecDNA formation11,15,30,31,32,34,35. In the FHCC samples, we found that WGD and chromothripsis were significantly associated with TP53 alteration (Extended Data Fig. 6c,d), indicative of its role in mediating genomic stability. However, many of the samples with ecDNA did not show evidence of chromothripsis or WGD (Extended Data Fig. 6e,f), indicating that there are other mechanisms of ecDNA formation after TP53 alteration.
ecDNA and malignant transformation
To better understand the potential relationship between ecDNA and the transition from HGD to EAC, we studied an individual patient in the FHCC cancer-outcome cohort (patient 391), whose WGS data were collected at four endoscopies over a seven-year period (Fig. 3a). At first, HGD was detected at two different locations within the Barrett’s oesophagus segment. Chromosomal ERBB2 amplification through breakage–fusion–bridge (BFB) cycles (Extended Data Fig. 7a) and TP53 alterations were present in these biopsies (Fig. 3b). An ecDNA (ecDNA-1), containing AP2B1, GAS2L2 and RASL10B, was only detected (Fig. 3b,c and Extended Data Fig. 7b) after 5.6 years. The lesions did not progress to EAC for another 6.5 months, at which point a second ecDNA (ecDNA-2) containing SOCS1, CIITA and RMI2 was detected (Fig. 3b,c and Extended Data Fig. 7c). SOCS1 is a suppressor of cytokine signalling, including interferon-γ36, which may foster escape from cytotoxic T cells37. CIITA is an immunomodulatory master transcription factor for antigen presentation38, and its translocation is immunosuppressive39. RMI2 is a component of the Bloom helicase complex, which is involved in homologous recombination and might have a role in lung cancer metastases40. A subsequent surgical resection of the tumour confirmed both ecDNA-1 and ecDNA-2, whereas the tissue that contained only ecDNA-1, TP53 alteration and chromosomal ERBB2 amplification remained HGD. These results suggest that multiple and ongoing focal amplification events occur in dysplastic tissues41,42, enhancing the fitness of a clone during malignant transformation.
a, Timeline of sample collection for FHCC CO patient 391 relative to patient age. Summary of the ecDNA status and windowed-histology status for four endoscopies, with the time interval between each indicated. The distance of the biopsy from the gastro-oesophageal junction (GEJ) is also shown. The two resection samples are labelled as E8 and C5. Two distinct species of ecDNA are labelled as ecDNA-1 and ecDNA-2. b, Inferred phylogeny of Barrett’s oesophagus samples from patient 391 across the four endoscopies, starting from TP53 alteration, with branching reporting the ecDNA formation events, annotated by the histological status of the sample (windowed). c, Left, structure of ecDNA-1, first detected in endoscopy 2, in which HGD was detected within ±1 cm. Right, structure of ecDNA-2, first detected in endoscopy 3, in which EAC was diagnosed and present within ±1 cm.
ecDNAs with common origins
To compare the fine structures of ecDNAs across multiple time points and biopsies from the same individual, we developed an amplicon similarity score ranging from 0 to 1 (Supplementary Fig. 1a–e and Supplementary Information). Genomically overlapping ecDNAs in different samples from the same patient showed a high similarity, consistent with a common origin (Supplementary Fig. 1e). All ten genomically overlapping ecDNA pairs from within the same FHCC individuals, reidentified between different biopsies, showed a significant similarity (P < 0.05) (Supplementary Table 5). Thus, ecDNAs detected in pre-cancer are frequently maintained through the transition to cancer, and genomically overlapping ecDNAs identified from multi-region sampling are likely to have a common origin. Together, these data suggest that ecDNA can be a truncal event in the formation and evolution of EAC.
Selection and evolution of ecDNAs
We detected a marked increase in the frequency of ecDNA in biopsies from patients with a cancer outcome before clinical detection of cancer, and even higher levels of ecDNA in biopsies or resections from later-stage cancers (Fig. 4a). To better understand these observations, we characterized 137 ecDNAs across all samples from 75 patients with ecDNA-positive Barrett’s oesophagus, Barrett’s-oesophagus-derived HGD or Barrett’s-oesophagus-adjacent EAC. ecDNA copy number was significantly higher in EAC samples than in pre-cancer samples (Fig. 4b; Mann–Whitney U test, P = 0.033, one-sided). Although the lengths of genomic regions captured on ecDNA were not significantly different between pre-cancer and EAC states (Extended Data Fig. 8; Mann–Whitney U test, P = 0.44, two-sided), the complexity of structural rearrangements in ecDNA-derived regions increased between pre-cancer and EAC (Fig. 4c; Mann–Whitney U test, P = 0.043, one-sided), suggesting a significant increase in the heterogeneity of ecDNA structures with the evolution of tumours. We next investigated copy number changes in eight pairs of clonal ecDNA in which the same ecDNAs (on the basis of amplicon similarity score) appeared in different sequencing biopsies from the same patient, and for which the biopsies also had windowed-histology data available (Supplementary Table 1). When both ecDNA occurrences were associated with the same histology, the ecDNA copy numbers were highly similar. However, if one sample was associated with a more severe histological status than the other, the ecDNA copy number was significantly higher in that sample (Fig. 4d; Mann–Whitney U test, P = 0.029, one-sided). These data suggest that ecDNA confers a strong selective advantage to the Barrett’s oesophagus clones that eventually progress to EAC, and that pre-cancer ecDNAs are subject to continued evolution during malignant transformation and progression, leading to increased heterogeneity and copy number.
a, Proportion of patients with ecDNA detected in any sample across all study cohorts. b, Maximum genomic copy number (CN) of ecDNA segments in pre-cancer samples and EAC (or EAC-linked for FHCC) samples, coloured by sample study source. c, Complexity score of focally amplified ecDNA-positive genomic regions for pre-cancer and EAC samples. d, For ecDNAs identified across multiple FHCC samples that were determined to be clonal on the basis of amplicon similarity, the increase in ecDNA copy number for each pair of clonal ecDNAs, separated by the difference in associated histology of the two samples, shows an association between increasing copy number and increasing histological severity. e, Number of distinct ecDNAs per sample, among the ecDNA-positive samples, from all combined sources of data. f, Comparative overlap of Barrett’s-oesophagus-associated oncogenes found on ecDNA in the four cohorts. g, For oncogenes recurrently detected on ecDNA in samples from different patients, the number of patients with a sample that has the listed oncogene included on ecDNA. h, Oncogene copy number for the focally amplified oncogene with the highest copy number on each unique focal amplification (ecDNA or non-ecDNA fsCNA) is significantly higher on ecDNA versus non-ecDNA fsCNA.
Twenty-six out of 83 (31%) ecDNA-positive samples from the combined study sources contained more than one species of ecDNA (Fig. 4e), enabling multiple oncogene amplifications. Multiple species of ecDNA could also be detected in Barrett’s oesophagus HGD samples from patients who progressed to EAC (Supplementary Table 3), raising the possibility that tumours might achieve subclonal ecDNA heterogeneity early on, and that competition between multiple distinct ecDNAs could have a role in the evolution of EAC.
Diversity of ecDNA-borne genes
We identified a large diversity of oncogenes on the ecDNAs, many of which were not detected on non-ecDNA focal amplifications (Extended Data Fig. 9a). ecDNAs were significantly enriched for oncogenes as compared to non-oncogenes (Extended Data Fig. 9b; Fisher’s exact test, P = 8.9 × 10−4, one-sided)—including oncogenes that are known to drive EAC, such as ERBB2, KRAS and MYC, which were recurrently detected on ecDNAs found in Barrett’s oesophagus and EAC across multiple cohorts (Fig. 4f,g and Supplementary Table 6). Furthermore, 33.1% of the ecDNAs contained multiple oncogenes on the same molecule (Extended Data Fig. 9c). ecDNAs contained 0.76 unique oncogenes per amplicon (97 oncogenes in 127 ecDNAs), compared to 0.52 (192/373) unique oncogenes per amplicon in non-extrachromosomal focal somatic copy number amplifications (fsCNAs), suggesting that ecDNA may allow a wider variety of oncogene amplifications.
ecDNA amplification was associated with a greater maximum oncogene copy number than other fsCNAs (Fig. 4h; distribution mean copy number = 11.6 and 21.3 for non-ecDNA and ecDNA, respectively, Mann–Whitney U test, P = 5.9 × 10−5, one-sided), with some ecDNA genes surpassing 100 copies. ecDNA also permitted greater diversity in maximum copy number than did non-ecDNA fsCNA (copy number variance = 687.9 versus 122.2 in non-ecDNA fsCNA, Levene’s test, P = 1.5 × 10−4). Notably, many ecDNA genes (79 in total) were associated with immunomodulation (Supplementary Table 7 and Extended Data Fig. 10a), and only 25 of the 79 were already present in the set of canonical oncogenes. The ecDNA amplified immunomodulatory genes had a significantly higher copy number compared to those on other fsCNAs (Extended Data Fig. 10b; Mann–Whitney U test, P = 4.1 × 10−3, one-sided).
A comparison of genomic regions that are predicted to be on ecDNA and oncogene intervals that are known to associate specifically with Barrett’s oesophagus and EAC (Supplementary Table 8 and Supplementary Fig. 2) showed a statistically significant overlap (ISTAT test43, P = 3.1 × 10−5; Supplementary Information), suggesting that—despite the high diversity of ecDNA-borne oncogenes—ecDNAs are positively selected in a manner that is specific to cancer type.
Discussion
Oncogene amplification on ecDNA enables tumours to evolve at an accelerated rate, which drives rapid resistance to therapy and contributes to shorter survival for patients2,3,5. It has been unclear whether ecDNA can contribute to the transformation of pre-cancer to cancer, or whether it is a later manifestation of tumour genomic instability. Here, in multiple cohorts of patients with Barrett’s oesophagus, we show that ecDNA appears in HGD, and that its presence is strongly associated with EAC progression.
Typical phylogenetic approaches to track cancer clonality assume chromosomal inheritance. In consequence, it has been challenging to infer the clonality and evolution of ecDNA-driven cancers. Our results show that in the evolution of tumours from pre-cancer to cancer, ecDNA confers a strong selective advantage to the Barrett’s oesophagus clones that eventually progress to EAC. The substantial heterogeneity in ecDNA-containing cancers might promote rapid and frequent branching of the phylogenetic tree, fostered by the non-chromosomal inheritance of ecDNA during cell division. Moreover, the increased prevalence and complexity of ecDNA structures in oesophageal cancer samples suggests ongoing selection and evolution during the formation and progression of tumours44.
Our results strongly suggest that ecDNAs usually arise in regions of HGD in patients with Barrett’s oesophagus, and nearly always in the context of TP53 alteration. These results complement previous reports that found that TP53 alteration and altered copy number might drive the transition from metaplasia to dysplasia10,13,14,45,46, showing the cooperative nature of various genetic and epigenetic alterations, and suggesting that ecDNA formation represents a particularly potent driver of transformation and could be an opportunity for specific therapeutic intervention. Moreover, our analysis of a single patient across time supports and extends a report46 suggesting that TP53 alteration results in polyploid tumours with multiple amplified or gained regions providing a reservoir for amplifying oncogenes.
Freed from Mendelian constraints, ecDNA amplifies a broader range of oncogenes, and their copy numbers increase rapidly and markedly in EAC, consistent with strong positive selection. Increased ecDNA heterogeneity may also enhance adaptation to changing conditions. Notably, the clonal selection and maintenance of immunomodulatory genes on ecDNA before cancer development could aid immune evasion. Together, these results indicate that ecDNA contributes to the development of cancer through several mechanisms. These findings shed light on how ecDNA can arise before the development of full-blown cancer, indicating that it is not simply a late manifestation of genome instability, and raise the possibility of earlier intervention or prevention for patients with ecDNA-containing tumours.
Methods
ecDNA detection and characterization
DNA copy number alterations were detected using CNVKit (ref. 47; FHCC and TCGA samples) and ASCAT (ref. 48; Cambridge samples). AmpliconSuite-pipeline (v.0.1203.12) was used to identify candidate seed regions for the detection and characterization of ecDNA using AmpliconArchitect v.1.2 (ref. 21) and AmpliconClassifier (v.0.4.13) (Supplementary Information and Fig. 1e), and circular visualizations of candidate ecDNA structures were generated using CycleViz (v.0.1.1). Amplicon complexity score was computed on the basis of the diversity of the amplicon structure decompositions output by AmpliconArchitect (Supplementary Information and Supplementary Fig. 3).
Statistical analysis
We used SciPy v.1.9.1 (ref. 49) to perform all statistical tests in the study, with the exception of the ecDNA region–oncogene overlap significance test, for which we used ISTAT v.1.0.0 (ref. 43 and Supplementary Information). When computing odds ratios, if any cell in the two-by-two table was zero, the Haldane correction50 was applied to every cell in the table. The significance of odds ratios and differences in event frequencies between groups were assessed by Fisher’s exact test. The default test type was two-sided in statistical tests, unless otherwise specified. For data represented in box plots, the centre line is the median, the box limits are the upper and lower quartiles and the whiskers are 1.5 times the interquartile range, or represent minimum or maximum values if there are no outliers.
Cambridge sample selection
We identified suitable patients from a prospective surveillance study of more than 3,000 patients with pre-cancerous lesions in a previous study13, in which the follow-up and methods for pathology reviewed were described. There was a minimum follow-up of 44 months (median 139, maximum 258) for the cohorts that did not progress to HGD or EAC, and a median follow-up of 57 months (range 0–249) for the dysplastic cohort. We analysed WGS data from 206 patients in cross-sectional Barrett’s oesophagus surveillance Cambridge cohorts with biopsy-validated Barrett’s oesophagus, including 27 patients with non-dysplastic Barrett’s oesophagus, 15 patients with LGD who never developed HGD or EAC during follow-up, 25 patients with HGD, 51 patients with early-stage EAC (AJCC stage I) and 88 patients with late-stage EAC (AJCC stage II–IV (Fig. 1a). Patients with low-grade Barrett’s oesophagus and high-grade Barrett’s oesophagus underwent surveillance at Cambridge University Hospitals NHS Trust and consented prospectively to a biomarker and genomic characterization study (Cell Determinants Biomarker, REC no. 01/149, BEST2 REC no. 10/H0308/71).
Patients included in the Cambridge surveillance cohorts were all treatment-naive—that is, the patients had received no neoadjuvant chemotherapy, radiation or ablation therapies—except for two of the patients with late-stage EAC (patients 167 and 155), who had received previous therapy, as detailed in Supplementary Table 1 and Extended Data Fig. 1a.
Strict selection criteria were implemented to ensure that only the highest-cellularity biopsies, with agreement of histological grade, were sequenced. Potential biopsies were placed into optimal cutting temperature compound and a single section was cut and stained with haematoxylin and eosin (H&E). These were reviewed by at least two consultant pathologists to assess the composition of the biopsy. All pathologists were blinded to the grade of the patient. Samples with no agreement were reviewed by a third pathologist to reach a consensus. Dysplastic samples for sequencing had to have a pathological cellularity for dysplasia of at least 30% and were labelled to be consistent with the highest pathology grade reported within the biopsy (tumour cellularity of 70% or higher for early-stage cancers). Non-dysplastic Barrett’s oesophagus biopsies had to contain intestinal metaplasia.
Patients with early- and late-stage EAC in the Cambridge cohorts were recruited for the EAC International Cancer Genome Consortium (ICGC) study, for which samples were collected through the UK-wide Oesophageal Cancer Classification and Molecular Stratification (OCCAMS, REC no. 10-H0305-1) consortium. For early-stage cancer samples, samples with a cellularity of 70% or higher were included, consistent with ICGC guidelines. Ethical approvals for these trials were from the East of England–Cambridge Central Research Ethics Committee. EAC samples were prospectively collected as endoscopic biopsies or resection specimens. All tissue samples were snap-frozen and blood or normal squamous epithelium (at least 5 cm from the tumour) was used as a germline reference as previously described13.
Barrett’s oesophagus research samples were collected at every 2 cm of the Barrett’s oesophagus segment at endoscopy, and snap-frozen. A snap-frozen section was taken from each Barrett’s oesophagus sample to determine the grade of dysplasia. Patients in the pre-cancer categories who received previous ablative treatment were excluded. Samples with squamous contamination were excluded.
Cambridge sequencing data
Sequencing was performed for cases with an estimated tumour purity of higher than 70%, as determined by a pathologist. WGS by Illumina (100–150-bp paired-end reads) was performed with 50-fold coverage for the tumour and 30-fold coverage for the matched germline control. Reads were then aligned with BWA-MEM51 to GRCh37 (1000 Genomes Project human_g1k_v37 with decoy sequences hs37d5). Aberrant cell fraction and ploidy were previously reported13 and were generated using ASCAT v.2.3 (ref. 48).
Detection of focal amplifications in the Cambridge cohort
Both Cambridge BAM files were aligned to GRCh37 (1000 Genomes Project human_g1k_v37 with decoy sequences hs37d5) using BWA-MEM v.0.7.17. Absolute copy number profiles were generated using ASCAT v.2.3 (ref. 48). Genomic regions with a total copy number greater than 4.5 and an interval size greater than 10 kbp were identified, merged and refined with the amplified_intervals.py script. Each seed region was given to AmpliconArchitect separately to improve runtime on each sample. AmpliconArchitect was run in the default explore mode to reconstruct amplicon structures and amplicons formed by the same regions were deduplicated on the basis of genomic overlap such that for overlapping AmpliconArchitect amplicons, the amplicon with the highest-level classification was kept (ranked by ecDNA, BFB, complex non-cyclic and then linear), with ties being broken by largest amplicon size.
Detection of focal amplifications in the TCGA cohort
We used the Dockerized AmpliconSuite-pipeline wrapper to detect focal amplifications in the TCGA cohort. The wrapper pipeline for seed detection incorporated CNVKit v.0.9.7 (ref. 47) run in unpaired mode to detect CNVs. The CNV calls were then provided with the amplified_intervals.py script and filtered on the basis of regions having a copy number greater than 4.5 and a size larger than 50 kbp to produce a set of seed regions. We used AmpliconArchitect to infer the architecture of amplicons, The pipeline was run on 20 TCGA-ESCA EAC tumour WGS BAM files, aligned to GRCh37, through the Institute for Systems Biology Cancer Genomics Cloud (https://isb-cgc.appspot.com/), which provides a cloud-based platform for TCGA data analysis.
FHCC sequencing data and annotations
Sequencing data for the FHCC study were previously published14. All research participants who contributed clinical data and biospecimens to this study provided written informed consent, subject to oversight by the Fred Hutchinson Cancer Center IRB Committee D (reg. ID 5619). All samples collected for the FHCC study were from patients who had not received treatment (treatment-naive). Reads were then aligned with BWA-MEM (v.0.6.2-r126)51 to GRCh37 (1000 Genomes Project human_g1k_v37 with decoy sequence hs37d5). BAM files underwent subsequent indel realignment with GATK IndelRealigner v.3.4-0-g7e26428 (ref. 52). Chromothripsis calls were derived from a previous study22. Genome-doubling (WGD) calls were derived from another previous study14. Purity and ploidy were also assessed as described previously14, using pASCAT v.2.1.
Detection of focal amplifications in the FHCC cohort
We used the AmpliconSuite-pipeline wrapper to detect focal amplifications in the FHCC cohort. The wrapper pipeline for seed detection incorporated CNVKit v.0.9.6 (ref. 47) run in tumour-normal mode to call somatic CNVs against the matched normal WGS samples for each patient (when multiple normal samples were available, one was selected arbitrarily). Normal samples also underwent the same pipeline in unpaired mode for stand-alone CNV detection. The CNV calls were then provided the amplified_intervals.py script and filtered on the basis of regions having a copy number greater than 4.3 (4.0 for normal samples) and size larger than 50 kbp (10 kbp for normal samples) to produce a set of seed regions. The wrapper then invoked AmpliconArchitect in default mode on the WGS BAM files to examine seed regions and profile the architecture of the focal amplifications. The resulting graph and cycles output files were provided to AmpliconClassifier v.0.4.13 to produce classifications of the AmpliconArchitect amplicons for ecDNA, BFB, complex non-cyclic and linear focal amplifications (Supplementary Information). AmpliconClassifier also specified BED files corresponding to the classified regions and annotated the identity of genes on the focal amplifications.
FHCC cohort histology
The histology data from FHCC are a re-analysis of the previously published cohort14. In brief, the biopsy samples that underwent WGS were not assessed for pathological diagnosis, by design. Instead, the pathological analysis was performed from adjacent-level biopsies from the oesophagus, as described before14. If a sequencing biopsy had a histology biopsy from the same level along the oesophagus (measured from the gastro-oesophageal junction), then it was denoted as having on-level histology. If a sequencing biopsy had a histology biopsy from within ±1 cm of the same level, it was denoted as having windowed histology. When multiple histology samples could be paired with the sequencing, the histology biopsy with the most severe disease state was assigned.
TP53 alteration analysis
In the FHCC cohort, TP53 status was determined from a previous study14 and we defined TP53 alteration as cases in which either single (+/−) or double (−/−) loss of TP53 was detected. In brief, for the FHCC cohort, mutations were defined as any moderate- to high-impact SNV or indel as reported by SNPeff53. Deletions of at least one exon, or structural variants affecting the TP53 coding sequence or splice sites, were also considered to disrupt TP53, as were copy number alterations that affected at least half of the exonic regions. All alterations were verified manually using IGV54 or Partek. For the Cambridge cohort, TP53 status was determined by identifying somatic coding variants (missense, frameshift, stop-gain or splice-site variants), using Strelka v.2.0.15 (ref. 55) and Variant Effect Predictor v.78 (ref. 56). Alteration was defined as one or more copies of TP53 being affected by a mutational event.
Selection of gene lists
Oncogenes were derived from a combination of the ONGene database57, as well as Barrett’s oesophagus and EAC driver genes listed in previous reports14,58,59. The complete list is provided in Supplementary Table 8. Immunomodulatory genes were derived from the HisgAtlas database60. When evaluating the presence of genes on ecDNA, the average gene copy number was required to be 4.5 or higher and the 5′ end intact.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Barrett’s oesophagus and HGD Cambridge UK cohort WGS data, histology and metadata have been previously published13, and WGS data are available through the European Genome-phenome Archive (EGA) under accession number EGAD00001006349. The EAC Cambridge UK cohort WGS data, histology and metadata were downloaded from the International Cancer Genome Consortium (ICGC; https://dcc.icgc.org/) under accession number EGAD00001002156. The FHCC cohort WGS samples, histology and metadata have been previously published14, and WGS data are available from the NCBI dbGaP database under accession number phs001912.v1.p1. All sequencing data, histology and metadata for TCGA were downloaded from the Genomic Data Commons (GDC; https://gdc.cancer.gov/) under accession number phs000178.v11.p8. We have uploaded the AmpliconArchitect and AmpliconClassifier output files to figshare at https://doi.org/10.6084/m9.figshare.21893826.
Code availability
We developed and used the following publicly available codebases: AmpliconArchitect (https://github.com/jluebeck/AmpliconArchitect), AmpliconClassifier (https://github.com/jluebeck/AmpliconClassifier), AmpliconSuite-pipeline (https://github.com/jluebeck/AmpliconSuite-pipeline) and CycleViz (https://github.com/jluebeck/CycleViz).
References
Turner, K. M. et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 543, 122–125 (2017).
Nathanson, D. A. et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 343, 72–76 (2014).
Kim, H. et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat. Genet. 52, 891–897 (2020).
Verhaak, R. G. W., Bafna, V. & Mischel, P. S. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat. Rev. Cancer 19, 283–288 (2019).
Wu, S., Bafna, V., Chang, H. Y. & Mischel, P. S. Extrachromosomal DNA: an emerging hallmark in human cancer. Annu. Rev. Pathol. 17, 367–386 (2022).
Lange, J. T. et al. The evolutionary dynamics of extrachromosomal DNA in human cancers. Nat. Genet. 54, 1527–1533 (2022).
Peters, Y. et al. Barrett oesophagus. Nat. Rev. Dis. Primers 5, 35 (2019).
Prasad, G. A., Bansal, A., Sharma, P. & Wang, K. K. Predictors of progression in Barrett’s esophagus: current knowledge and future directions. Am. J. Gastroenterol. 105, 1490 (2010).
Alnasser, S. et al. Predictors of dysplastic and neoplastic progression of Barrett’s esophagus. Can. J. Surg. 62, 93 (2019).
Li, X. et al. Temporal and spatial evolution of somatic chromosomal alterations: a case-cohort study of Barrett’s esophagus. Cancer Prev. Res. 7, 114–127 (2014).
Nones, K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 5, 5224 (2014).
Killcoyne, S. et al. Genomic copy number predicts esophageal cancer years before transformation. Nat. Med. 26, 1726–1732 (2020).
Katz-Summercorn, A. C. et al. Multi-omic cross-sectional cohort study of pre-malignant Barrett’s esophagus reveals early structural variation and retrotransposon activity. Nat. Commun. 13, 1407 (2022).
Paulson, T. G. et al. Somatic whole genome dynamics of precancer in Barrett’s esophagus reveals features associated with disease progression. Nat. Commun. 13, 2300 (2022).
Campbell, P. J. et al. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Hung, K. L. et al. ecDNA hubs drive cooperative intermolecular oncogene expression. Nature 600, 731–736 (2021).
Yi, E. et al. Live-cell imaging shows uneven segregation of extrachromosomal DNA elements and transcriptionally active extrachromosomal DNA hubs in cancer. Cancer Discov. 12, 468–483 (2022).
Wu, S. et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature 575, 699–703 (2019).
Morton, A. R. et al. Functional enhancers shape extrachromosomal oncogene amplifications. Cell 179, 1330–1341 (2019).
Hung, K. L., Mischel, P. S. & Chang, H. Y. Gene regulation on extrachromosomal DNA. Nat. Struct. Mol. Biol. 29, 736–744 (2022).
Deshpande, V. et al. Exploring the landscape of focal amplifications in cancer using AmpliconArchitect. Nat. Commun. 10, 392 (2019).
Hadi, K. et al. Distinct classes of complex structural variation uncovered across thousands of cancer genome graphs. Cell 183, 197–210 (2020).
Shale, C. et al. Unscrambling cancer genomes via integrated analysis of structural variation and copy number. Cell Genomics 2, 100112 (2022).
Ng, A. W. T. et al. Rearrangement processes and structural variations show evidence of selection in oesophageal adenocarcinomas. Commun. Biol. 5, 335 (2022).
Stachler, M. D. et al. Genomic signatures of past and present chromosomal instability in the evolution of Barrett’s esophagus to esophageal adenocarcinoma. Preprint at bioRxiv https://doi.org/10.1101/2021.03.26.437288 (2023).
Rice, T. W., Patil, D. T. & Blackstone, E. H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann. Cardiothorac. Surg. 6, 119 (2017).
The Cancer Genome Atlas Research Network.Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017).
Eischen, C. M. Genome stability requires p53. Cold Spring Harb. Perspect. Med. 6, a026096 (2016).
Hanel, W. & Moll, U. Links between mutant p53 and genomic instability. J. Cell. Biochem. 113, 433–439 (2012).
Shoshani, O. et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 591, 137–141 (2020).
Rosswog, C. et al. Chromothripsis followed by circular recombination drives oncogene amplification in human cancer. Nat. Genet. 53, 1673–1685 (2021).
Ly, P. et al. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat. Genet. 51, 705 (2019).
Dewhurst, S. M. et al. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 4, 175–185 (2014).
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Umbreit, N. T. et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 368, eaba0712 (2020).
Seymour, G. J. et al. Immunohistologic analysis of the inflammatory infiltrates associated with osseointegrated implants. Int. J. Oral Maxillofac. Implants 4, 191–198 (1989).
Lawson, K. A. et al. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature 586, 120–126 (2020).
Kobayashi, K. S. & Van Den Elsen, P. J. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat. Rev. Immunol. 12, 813–820 (2012).
Steidl, C. et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 471, 377–383 (2011).
Zhan, W. et al. RMI2 plays crucial roles in growth and metastasis of lung cancer. Signal Transduct. Target. Ther. 5, 188 (2020).
Schmidt, M. et al. Evolutionary dynamics in Barrett oesophagus: implications for surveillance, risk stratification and therapy. Nat. Rev. Gastroenterol. Hepatol. 19, 95–111 (2022).
Zahir, N., Sun, R., Gallahan, D., Gatenby, R. A. & Curtis, C. Characterizing the ecological and evolutionary dynamics of cancer. Nat. Genet. 52, 759–767 (2020).
Sarmashghi, S. & Bafna, V. Computing the statistical significance of overlap between genome annotations with iStat. Cell Syst. 8, 523 (2019).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613–628 (2017).
Redston, M. et al. Abnormal TP53 predicts risk of progression in patients with Barrett’s esophagus regardless of a diagnosis of dysplasia. Gastroenterology 162, 468–481 (2022).
Baslan, T. et al. Ordered and deterministic cancer genome evolution after p53 loss. Nature 608, 795–802 (2022).
Talevich, E., Shain, A. H., Botton, T. & Bastian, B. C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Van Loo, P. et al. Allele-specific copy number analysis of tumors. Proc. Natl Acad. Sci. USA 107, 16910–16915 (2010).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Haldane, J. B. S. The mean and variance of the moments of chi-squared when used as a test of homogeneity, when expectations are small. Biometrika 29, 133–134 (1940).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at arXiv https://doi.org/10.48550/arXiv.1303.3997 (2013).
Depristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–501 (2011).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 6, 80–92 (2012).
Thorvaldsdóttir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013).
Kim, S. et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat. Methods 15, 591–594 (2018).
McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 2891 (2016).
Liu, Y., Sun, J. & Zhao, M. ONGene: a literature-based database for human oncogenes. J. Genet. Genomics 44, 119–121 (2017).
Frankell, A. M. et al. The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat. Genet. 51, 506–516 (2019).
Stachler, M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 47, 1047–1055 (2015).
Liu, Y. et al. HisgAtlas 1.0: a human immunosuppression gene database. Database 2017, bax094 (2017).
Acknowledgements
We thank the many individuals who donated their tissue samples to scientific research, without whom this study could not have taken place. This work was delivered as part of the eDyNAmiC team supported by the Cancer Grand Challenges partnership funded by Cancer Research UK (CRUK) (P.S.M. and H.Y.C., CGCATF-2021/100012; V.B. and J.L., CGCATF-2021/100025; S.W., CGCATF-2021/100023; and R.G.W.V., CGCATF-2021/100016) and the National Cancer Institute (P.S.M. and H.Y.C., OT2CA278688; V.B. and J.L., OT2CA278635; S.W., OT2CA278683; and R.G.W.V., OT2CA278649). P.S.M. is the eDyNAmiC team lead and co-authors R.G.W.V., H.Y.C., S.W. and V.B. are members of the eDyNAmiC team. This study was also supported by a grant from the National Brain Tumour Society (P.S.M.) and National Institutes of Health (NIH) R01-CA238379 (P.S.M.). V.B. and J.L. were supported in part by grants U24CA264379 and R01GM114362 from the NIH. S.W. is a scholar of and is supported by the Cancer Prevention and Research Institute of Texas (RR210034). The Cambridge research (R.C.F., A.W.T.N. and S.J.) was funded by a programme grant from the Medical Research Council and from CRUK. The work also received infrastructure support from the NIHR Biomedical Research Centre and the CRUK Experimental Cancer Research Centre. A.C.K.-S. was funded by CRUK through a clinical research fellowship. Research at FHCC (T.G.P., B.J.R., C.A.S., P.C.G. and X.L.) was funded by NIH grants P01 CA91955 and P30 CA015704. H.K. is supported by the Brain Korea 21 Four Project, a Korean Ministry of Food and Drug Safety grant (21153MFDS607) and Korean Ministry of Science and ICT grants (NRF-2019R1A5A2027340 and NRF-2022M3C1A309202211). R.G.W.V. acknowledges support from NIH–NCI grants R01 CA237208, R21 CA256575 and R33 CA236681 and a Cancer Center Support grant P30 CA034196, as well as NIH–NINDS grant R21 NS114873. Work in the L.B.A. laboratory (L.B.A. and Y.H.) was supported by NIH grants R01ES030993-01A1 and R01ES032547-01 and a Cancer Grand Challenges Mutographs team award funded by CRUK (C98/A24032). L.B.A. is also personally supported by a Packard Fellowship for Science and Engineering. This work was also supported by the PreCancer Genome Atlas (PCGA) project with core funding from UC San Diego NCI P30 (P30 CA023100; S.M.L.), and in part by the SU2C-AACR-DT25-17 and US NIH grants R01DE026644, P30 CA023100, HHSN261201200031I and UG1CA242596. We also thank G. Devonshire, who ran the data-analysis pipeline for the Cambridge sequencing data. The oesophagus illustration shown in our study was created by scientific illustrator Tami Tolpa (Tolpa Studios, https://www.tolpa.com/).
Author information
Authors and Affiliations
Contributions
J.L., R.C.F., T.G.P., P.C.G., S.W., V.B. and P.S.M. designed and conceived the analyses. R.C.F. led the Cambridge University UK team and T.G.P. led the FHCC team. The Cambridge data were analysed by J.L., A.W.T.N., A.C.K.-S., S.J. and R.C.F. The FHCC data were analysed by J.L., P.C.G., X.L., C.A.S., Y.H., L.B.A., C.C.M., B.J.R. and T.G.P. The TCGA data were analysed by J.L., H.K. and R.G.W.V. Computational methods introduced in the manuscript were conceived by J.L. and V.B. J.L., S.W., V.B. and P.S.M. wrote the manuscript, and R.C.F., A.W.T.N, T.G.P., P.C.G. and H.Y.C. provided input during the writing of the paper. All authors provided feedback on the analyses, edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.S.M. is a co-founder, chairs the scientific advisory board (SAB) of and has equity interest in Boundless Bio. P.S.M. is also an advisor with equity for Asteroid Therapeutics and is an advisor to Sage Therapeutics. V.B. is a co-founder, consultant, SAB member and has equity interest in Boundless Bio and Abterra, and the terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. S.W. is a member of the SAB of Dimension Genomics. H.Y.C. is a co-founder of Accent Therapeutics, Boundless Bio, Cartography Bio, and Orbital Therapeutics, and is an advisor to 10X Genomics, Arsenal Biosciences, Chroma Medicine, and Spring Discovery. R.C.F. is named on patents related to Cytosponge and associated assays that were licensed to Covidien (now Medtronic). R.C.F. has founder shares (<3%) in Cyted. R.G.W.V. is a co-founder of Boundless Bio and an advisor to Stellanova Therapeutics and NeuroTrials. L.B.A. is a compensated consultant and has equity interest in io9. His spouse is an employee of Biotheranostics. L.B.A. is an inventor on the US patent 10,776,718 for source identification by non-negative matrix factorization. L.B.A. also declares provisional patent applications for ‘Clustered mutations for the treatment of cancer’ (US provisional application serial number 63/289,601) and ‘Artificial intelligence architecture for predicting cancer biomarker’ (serial number 63/269,033). S.M.L. is a co-founder of io9, and also declares a provisional patent application for ‘Methods and biomarkers in cancer’ (US provisional application serial number 114198-1160). J.L. is a part-time paid consultant for Boundless Bio, and the terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. P.S.M., V.B., J.L. and S.W. declare a patent application related to this work: ‘Methods and compositions for detecting ecDNA’ (US patent application number 17/746,748). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Lorenzo Ferri, David Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Oncoprint tables for the Cambridge data.
a, Oncoprint table for samples from Cambridge patient with Barrett’s oesophagus and EAC segregated by histology type showing ecDNA status, histology or cancer stage (if applicable) TP53 alteration (by mutational analysis, involving at least one copy), BFB status, other fsCNA (non-BFB, non-ecDNA) status and prior therapy (chemotherapy or radiation) on the tumours in patients with cancer. b, Proportion of Cambridge EAC tumour samples with ecDNA separated by tumour stage I versus stage II or higher.
Extended Data Fig. 2 Oncoprint tables for the FHCC cancer-outcome data.
Oncoprint tables of samples from FHCC CO patient WGS samples encoding ecDNA status, BFB status, other fsCNA (non-BFB, non-ecDNA) status, TP53 alteration (at least one gene copy affected), WGD status and chromothripsis status, as well as on-level and windowed histology for each time point and both upper and lower oesophageal samples for time points TP-1 and TP-2. Maximum histology from any histology biopsy is shown at the bottom of each time point. Asterisk indicates cancer diagnosis made at next endoscopy since biopsies from the diagnostic EAC endoscopy were unavailable for CO patient ID 772 and lacked sufficient DNA for CO patient ID 568, so biopsies from the penultimate endoscopy were substituted (occurring 1.44 and 8.16 months after TP-2 for patients 568 and 772, respectively).
Extended Data Fig. 3 Oncoprint tables for the FHCC non-cancer-outcome data.
Oncoprint tables of FHCC NCO patient WGS samples encoding ecDNA status, BFB status, other fsCNA (non-BFB, non-ecDNA) status, TP53 alteration (at least one gene copy affected), WGD status and chromothripsis status, as well as on-level and windowed histology for each time point and both upper and lower oesophageal samples for time points TP-1 and TP-2. Maximum histology from any histology biopsy is shown at the bottom of each time point.
Extended Data Fig. 4 Analysis of ecDNA evolution.
The KRAS-bearing ecDNA focal amplification detected in biopsies from FHCC NCO patient 303 at time point TP-1 and time point TP-2. Amplicon similarity analysis reveals a common origin of the ecDNA, and ecDNA copy number and complexity increased during the 1.61 years between samples. P value assessed against a beta-distribution model fit to distribution of similarity scores among genomically overlapping focal amplifications from independent samples (Supplementary Information).
Extended Data Fig. 5 Oncoprint tables for FHCC non-cancer-outcome long-term follow-ups.
a, Oncoprint tables of biopsies from NCO patients from the FHCC cohort with long-term follow-ups (in orange, collected median 9.6 years after TP-2). b, Distribution of FHCC NCO follow-up durations from TP-2 to the time at which the patient was last known to be alive (top, mean = 13.9 years) or TP-2 to death (bottom, mean = 8.6 years).
Extended Data Fig. 6 Association of ecDNA with other genomic features.
a, Association of ecDNA presence and TP53 status in biopsies from patients from the FHCC cohort. b, Association of ecDNA presence and TP53 status in samples from patients from the Cambridge cohort, respectively for FHCC and Cambridge. c, Proportion of FHCC samples with WGD separated by TP53 alteration status. d, Proportion of FHCC samples with chromothripsis separated by TP53 alteration status. e, Proportion of TP53 alteration FHCC samples with ecDNA, separated by WGD status. f, Proportion of TP53 alteration FHCC samples with ecDNA, separated by chromothripsis status. All statistical differences in frequencies were assessed by one-sided Fisher’s exact test.
Extended Data Fig. 7 Focal amplification evolution.
a, Barrett’s oesophagus segment samples from patient 391 show conserved focal amplification of BFB and emergence of ecDNA between time points TP-1 and TP-2. b, The structure of ecDNA-1 detected in the lower pre-cancer sample from TP-2 in patient 391, in which HGD was in the histology window, and an identical structure derived from the adenocarcinoma resection. c, The structure of ecDNA-2, detected in the upper sample from TP-2 in patient 391, in which EAC was present in the histology window, and an identical structure derived from the adenocarcinoma resection. Amplicon similarity analysis of ecDNA-1 and -2 reveals common origins of the structures.
Extended Data Fig. 8 Sizes of intervals captured on ecDNA.
The length of predicted genomic intervals captured on ecDNA, visualized on a log10 scale, for each distinct ecDNA in the combined cohorts, compared by pre-cancer versus EAC (Mann–Whitney U test, two-sided).
Extended Data Fig. 9 Frequencies of ecDNA-borne oncogenes.
a, For oncogenes detected on ecDNA in samples from at least one patient, the number of patients with at least one sample having the oncogene listed on ecDNA, and the frequency of that gene on other types of focal amplifications. b, Proportion of the set of possible unique genes on ecDNA, separated by oncogene status. Difference assessed by one-sided Fisher’s exact test. c, Distribution of the number of oncogenes on individual ecDNA.
Extended Data Fig. 10 Frequencies of ecDNA-borne immunomodulatory genes.
a, For immunomodulatory-associated genes detected on ecDNA, the number of patients with at least one sample having the gene listed on ecDNA, and the frequency of that gene on other focal amplifications as well. b, Copy number for the highest copy number focally amplified immunomodulatory-associated gene in each unique amplicon that was ecDNA or non-ecDNA fsCNA. ecDNAs show a significantly higher copy number of immunomodulatory-associated genes on ecDNA versus non-ecDNA fsCNA (Mann–Whitney U test, one-sided).
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Supplementary Figures 1-4.
Supplementary Table 1
This file contains sample metadata and histology labels.
Supplementary Table 2
This file contains FHCC patient age at sampling and additional histology labels.
Supplementary Table 3
This file contains focal amplification classifications for all samples in the study.
Supplementary Table 4
This file contains properties related to the sample purity, ploidy, cellularity, coverage for the FHCC and Cambridge samples.
Supplementary Table 5
This file contains focal amplification similarity scores for ecDNAs, and all focal amplifications.
Supplementary Table 6
This file contains genes carried on all focal amplifications.
Supplementary Table 7
This file contains a list of immunomodulatory genes used in this study.
Supplementary Table 8
This file contains a list of oncogenes used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luebeck, J., Ng, A.W.T., Galipeau, P.C. et al. Extrachromosomal DNA in the cancerous transformation of Barrett’s oesophagus. Nature 616, 798–805 (2023). https://doi.org/10.1038/s41586-023-05937-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05937-5
This article is cited by
-
Full-spectral genome analysis of natural killer/T cell lymphoma highlights impacts of genome instability in driving its progression
Genome Medicine (2024)
-
Machine learning-based extrachromosomal DNA identification in large-scale cohorts reveals its clinical implications in cancer
Nature Communications (2024)
-
Extrachromosomal DNA in cancer
Nature Reviews Cancer (2024)
-
Imaging extrachromosomal DNA (ecDNA) in cancer
Histochemistry and Cell Biology (2024)
-
A decade of the Oesophageal Cancer Clinical and Molecular Stratification Consortium
Nature Medicine (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.