Abstract
The central amygdala (CeA) is implicated in a range of mental processes including attention, motivation, memory formation and extinction and in behaviours driven by either aversive or appetitive stimuli1,2,3,4,5,6,7. How it participates in these divergent functions remains elusive. Here we show that somatostatin-expressing (Sst+) CeA neurons, which mediate much of CeA functions3,6,8,9,10, generate experience-dependent and stimulus-specific evaluative signals essential for learning. The population responses of these neurons in mice encode the identities of a wide range of salient stimuli, with the responses of separate subpopulations selectively representing the stimuli that have contrasting valences, sensory modalities or physical properties (for example, shock and water reward). These signals scale with stimulus intensity, undergo pronounced amplification and transformation during learning, and are required for both reward and aversive learning. Notably, these signals contribute to the responses of dopamine neurons to reward and reward prediction error, but not to their responses to aversive stimuli. In line with this, Sst+ CeA neuron outputs to dopamine areas are required for reward learning, but are dispensable for aversive learning. Our results suggest that Sst+ CeA neurons selectively process information about differing salient events for evaluation during learning, supporting the diverse roles of the CeA. In particular, the information for dopamine neurons facilitates reward evaluation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article and its Supplementary Information. Supporting data can be downloaded at https://figshare.com/articles/dataset/data_for_paper/21956900. Source data are provided with this paper.
Code availability
Source code can be downloaded at https://figshare.com/articles/software/code_for_paper/21956801.
References
LeDoux, J. E. The amygdala and emotion: a view through fear. in The Amygdala: A Functional Analysis 2nd edn (ed. Aggleton, J. P.) 289–310 (Oxford Univ. Press, 2000).
Maren, S. Building and burying fear memories in the brain. Neuroscientist 11, 89–99 (2005).
Fadok, J. P., Markovic, M., Tovote, P. & Luthi, A. New perspectives on central amygdala function. Curr. Opin. Neurobiol. 49, 141–147 (2018).
Balleine, B. W. & Killcross, S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 29, 272–279 (2006).
Everitt, B. J., Cardinal, R. N., Parkinson, J. A. & Robbins, T. W. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann. N.Y. Acad. Sci. 985, 233–250 (2003).
Li, B. Central amygdala cells for learning and expressing aversive emotional memories. Curr. Opin. Behav. Sci. 26, 40–45 (2019).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Herry, C. & Johansen, J. P. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 17, 1644–1654 (2014).
Roesch, M. R., Esber, G. R., Li, J., Daw, N. D. & Schoenbaum, G. Surprise! Neural correlates of Pearce–Hall and Rescorla–Wagner coexist within the brain. Eur. J. Neurosci. 35, 1190–1200 (2012).
Duvarci, S. & Pare, D. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014).
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).
Duvarci, S., Popa, D. & Pare, D. Central amygdala activity during fear conditioning. J. Neurosci. 31, 289–294 (2011).
Iordanova, M. D., Deroche, M. L., Esber, G. R. & Schoenbaum, G. Neural correlates of two different types of extinction learning in the amygdala central nucleus. Nat. Commun. 7, 12330 (2016).
Calu, D. J., Roesch, M. R., Haney, R. Z., Holland, P. C. & Schoenbaum, G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron 68, 991–1001 (2010).
Shabel, S. J. & Janak, P. H. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc. Natl Acad. Sci. USA 106, 15031–15036 (2009).
Yu, K. et al. The central amygdala controls learning in the lateral amygdala. Nat. Neurosci. 20, 1680–1685 (2017).
Steinberg, E. E. et al. Amygdala–midbrain connections modulate appetitive and aversive learning. Neuron https://doi.org/10.1016/j.neuron.2020.03.016 (2020).
Douglass, A. M. et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 20, 1384–1394 (2017).
Yu, K., Garcia da Silva, P., Albeanu, D. F. & Li, B. Central amygdala somatostatin neurons gate passive and active defensive behaviors. J. Neurosci. 36, 6488–6496 (2016).
Sadacca, B. F., Rothwax, J. T. & Katz, D. B. Sodium concentration coding gives way to evaluative coding in cortex and amygdala. J. Neurosci. 32, 9999–10011 (2012).
Cassell, M. D. & Gray, T. S. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J. Comp. Neurol. 281, 320–333 (1989).
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013).
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010).
Penzo, M. A., Robert, V. & Li, B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci. 34, 2432–2437 (2014).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature https://doi.org/10.1038/nature13978 (2015).
Hartley, N. D. et al. Dynamic remodeling of a basolateral-to-central amygdala glutamatergic circuit across fear states. Nat. Neurosci. 22, 2000–2012 (2019).
Kim, J., Zhang, X., Muralidhar, S., LeBlanc, S. A. & Tonegawa, S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479 (2017).
Zhou, M. et al. A central amygdala to zona incerta projection is required for acquisition and remote recall of conditioned fear memory. Nat. Neurosci. 21, 1515–1519 (2018).
Shrestha, P. et al. Amygdala inhibitory neurons as loci for translation in emotional memories. Nature 586, 407–411 (2020).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).
Venniro, M. et al. Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc. Natl Acad. Sci. USA 117, 8126–8134 (2020).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Vankova, M., Arluison, M., Leviel, V. & Tramu, G. Afferent connections of the rat substantia nigra pars lateralis with special reference to peptide-containing neurons of the amygdalo-nigral pathway. J. Chem. Neuroanat. 5, 39–50 (1992).
Fudge, J. L. & Haber, S. N. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience 97, 479–494 (2000).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162, 622–634 (2015).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Ogawa, S. K., Cohen, J. Y., Hwang, D., Uchida, N. & Watabe-Uchida, M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 8, 1105–1118 (2014).
Lau, B., Monteiro, T. & Paton, J. J. The many worlds hypothesis of dopamine prediction error: implications of a parallel circuit architecture in the basal ganglia. Curr. Opin. Neurobiol. 46, 241–247 (2017).
Schultz, W. Dopamine reward prediction error coding. Dialogues Clin. Neurosci. 18, 23–32 (2016).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Deng, H. et al. A genetically defined insula–brainstem circuit selectively controls motivational vigor. Cell 184, 6344–6360 (2021).
Zhang, X. et al. Genetically identified amygdala–striatal circuits for valence-specific behaviors. Nat. Neurosci. 24, 1586–1600 (2021).
Zhang, X. & Li, B. Population coding of valence in the basolateral amygdala. Nat. Commun. 9, 5195 (2018).
Paton, J. J., Belova, M. A., Morrison, S. E. & Salzman, C. D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870 (2006).
Murray, J. E. et al. Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nat. Commun. 6, 10088 (2015).
Lingawi, N. W. & Balleine, B. W. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J. Neurosci. 32, 1073–1081 (2012).
Wang, Y. et al. Multimodal mapping of cell types and projections in the central nucleus of the amygdala. eLife 12, e84262 (2023).
Giovanniello, J. et al. A central amygdala–globus pallidus circuit conveys unconditioned stimulus-related information and controls fear learning. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.2090-20.2020 (2020).
He, M. et al. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 73, 35–48 (2012).
Zeng, W. B. et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol. Neurodegener. 12, 38 (2017).
Li, X. et al. Serotonin receptor 2c-expressing cells in the ventral CA1 control attention via innervation of the Edinger–Westphal nucleus. Nat. Neurosci. 21, 1239–1250 (2018).
Stephenson-Jones, M. et al. A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 (2016).
Xiong, Q., Znamenskiy, P. & Zador, A. M. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature 521, 348–351 (2015).
Znamenskiy, P. & Zador, A. M. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013).
Xiao, X. et al. A genetically defined compartmentalized striatal direct pathway for negative reinforcement. Cell 183, 211–227 (2020).
Keyes, P. C. et al. Orchestrating opiate-associated memories in thalamic circuits. Neuron 107, 1113–1123 (2020).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Pnevmatikakis, E. A. et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89, 285–299 (2016).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7, e28728 (2018).
Gallistel, C. R., Fairhurst, S. & Balsam, P. The learning curve: implications of a quantitative analysis. Proc. Natl Acad. Sci. USA 101, 13124–13131 (2004).
Sheintuch, L. et al. Tracking the same neurons across multiple days in Ca2+ imaging data. Cell Rep. 21, 1102–1115 (2017).
Rozeske, R. R. et al. Prefrontal-periaqueductal Gray–Projecting neurons mediate context fear discrimination. Neuron 97, 898–910 (2018).
Cunningham, J. P. & Yu, B. M. Dimensionality reduction for large-scale neural recordings. Nat. Neurosci. 17, 1500–1509 (2014).
Allen, W. E. et al. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 253 (2019).
Gao, Z. et al. A cortico-cerebellar loop for motor planning. Nature 563, 113–116 (2018).
Li, N., Daie, K., Svoboda, K. & Druckmann, S. Robust neuronal dynamics in premotor cortex during motor planning. Nature 532, 459–464 (2016).
Kim, C. K. et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328 (2016).
Acknowledgements
We thank R. Sharma for technical assistance and members of the Li laboratory for helpful discussions. This work was supported by grants from the National Institutes of Health (NIH) (R01MH101214, R01MH108924 and R01NS104944, B.L.), the Human Frontier Science Program (RGP0015/2016, B.L.), the Wodecroft Foundation (B.L.), the Cold Spring Harbor Laboratory and Northwell Health Affiliation (B.L.), the Feil Family Neuroscience Endowment (B.L.) and OFIRG20nov-0030 from the National Medical Research Council of Singapore (Y.F.).
Author information
Authors and Affiliations
Contributions
T.Y., K.Y. and B.L. conceived and designed the experiments. T.Y. built the wide-field microscope for in vivo imaging with GRIN lenses and conducted in vivo imaging experiments and imaging data analysis. K.Y. conducted in vivo optogenetic inhibition, tracing and fibre photometry experiments. X.Z. and X.X. wrote MATLAB (2017a) code for some of the imaging analysis. X.C. developed the AAVs for retrograde targeting of CeA neurons. Y.F. developed the Slc6a3Flp mouse line. T.Y. and B.L. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
X.C. (together with Q.W. and G.N.) has filed a patent covering some reagents used in this study (US application: PCT/US2022/07356; Stanford file: S21-124). All other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Richard Palmiter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
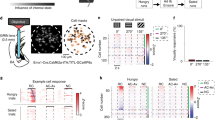
Extended Data Fig. 1 Characterization of Sst+ CeA neuronal responses.
a, A schematic of the experimental setup and the approach. b, A raw image of the field of view under a GRIN lens. c, A confocal histological image of a coronal brain section from a representative mouse used for the imaging experiments, showing GCaMP6 expression in Sst+ CeA neurons and the location of GRIN lens implantation. d, Schematics showing the placement of GRIN lens implants in the mice (n = 16 mice) used for the imaging experiments. Note that some mice were used in more than one experiment, as indicated. e, Four example neurons showing selective responses to different stimuli. f, Heat-maps of the responses of all neurons to different stimuli. Each row represents the activities of one neuron. g, The first six principle components (PC) (explain 84% of the variance) and hierarchical clustering dendrogram showing the relationship of each neuron within the five clusters. h, Average responses of each cluster to different stimuli (n = 199 neurons/4 mice). i & j, Correlation coefficient matrixes of the responses of all neurons for each stimulus pair. k, The spatial locations of individual extracted neurons in the field of view (FOV) in the CeA of a representative mouse. Sst+ neurons excited by different stimuli are color coded. l, Cumulative probability distributions of the pairwise distances (measured as centroid distances) of neurons excited by different stimuli, as indicated, in the FOV. The distributions of the pairwise distances were significantly different between groups (water-excited neurons vs. sucrose-excited neurons, ****P = 1.33e-04; water-excited neurons vs. food-excited neurons, ****P = 1.06e-12; water-excited neurons vs. shock-excited neurons, ****P = 6.28e-13; sucrose-excited neurons vs. food-excited neurons, ****P = 1.39e-05; sucrose-excited neurons vs. shock-excited neurons, ****P = 2.40e-06; food-excited neurons vs. shock-excited neurons, n.s., nonsignificant, P = 0.34; Kolmogorov-Smirnov test). Data from 4 mice were pooled together (n = 243 cells/4 mice). m, Left: pie chart of the percentage distributions of Sst+ CeA neurons in naïve mice, showing those selectively excited by water or sound, or neither stimulus (nonresponsive). Right: the fractions of sound- or water-excited neurons in individual mice (n = 239 neurons/6 mice, *P = 0.0224, paired t-test). Sound intensity, 70 dB. n, Trial-by-trial activities of a representative water-excited neuron and the first 5 licking responses of the mouse in response to water delivery. Both the neural and the licking responses are aligned to the onset of water presentation (dashed line). o, The timing of increase in activity in the water-excited neuron shown in n, and the timing of increase in licking response of the corresponding mouse after the onset of water delivery, as determined by change-point analysis (Methods). Black dots represent the change-points. p, Quantification of the difference between neural and behavioral change-points, for all water-excited neurons and the corresponding mice. Values above zero indicate that neural responses lag behind behavioral responses. q & r, Histogram showing the distribution of water-excited neurons based on the trial-by-trial Pearson correlation coefficients between their activities and the licking rates of the mouse in the 3-s (q) or 10-s (r) time window immediately after the onset of water delivery (n = 10, r (8) = 0.64, P = 0.046; P > 0.05 when −0.64 < r < 0.64; t-test). s–x, Data acquired during the conditioning phase. s, Trial-by-trial activities of a representative CSWA-excited neuron and the first 5 licking responses of the mouse in response to CSWA. Both the neural and the licking responses are aligned to CSWA onset. t, The timing of increase in activity in the neuron shown in s, and the timing of increase in licking response of the corresponding mouse after CSWA onset, as determined by change-point analysis (Methods). Black dots represent the change-points. u, Quantification of the difference between neural and behavioral change-points, for all the responsive neurons and the corresponding mice. Values above zero indicate that neural responses lag behind behavioral responses. v, Histogram showing the distribution of neurons based on trial-by-trial Pearson correlation coefficients between their average activities and the licking rates of the mouse in the 2.5-s time window immediately after CSWA onset. w, Histogram showing the distribution of neurons based on Pearson correlation coefficients between their USWA responses and the CSWA-induced licking rates of the mouse in subsequent trials (n = 19, r(17) = 0.46, P = 0.047; P > 0.05 when −0.46 < r < 0.46; t-test). x, Histogram showing the distribution of neurons based on Pearson correlation coefficients between their USWA responses (in three-trial rolling averages) and the CSWA-induced licking rates of the mouse (in the corresponding three-trial rolling averages) (n = 18, r(16) = 0.47, P = 0.049; P > 0.05 when −0.47 < r < 0.47; t-test). y-bb, Data acquired during the retrieval phase. y, Quantification of the difference between neural and behavioral change-points, for all the neurons excited by CSWA and the corresponding mice. z, Histogram showing the distribution of neurons based on trial-by-trial Pearson correlation coefficients between their average activities and the licking rates of the mouse in the 3-s time window immediately after CSWA onset (n = 20, r(18) = 0.45, P = 0.046; P > 0.05 when −0.45 < r < 0.45; t-test). aa, Quantification of the difference between neural and behavioral change-points, for all the neurons excited by CSSH and the corresponding mice. bb, Histogram showing the distribution of neurons based on trial-by-trial Pearson correlation coefficients between their average activities and the eye size change of the mouse in the 3-s time window immediately after CSSH onset (n = 15, r(13) = 0.52, P = 0.047; P > 0.05 when −0.52 < r < 0.52; t-test). cc-ff, data from the go/no-go task. cc, Quantification of the difference between neural and behavioral change-points, for all the neurons excited by CSSU and the corresponding mice in hit trials. dd, Histogram showing the distribution of neurons based on trial-by-trial Pearson correlation coefficients between their average activities and the licking rates of the mouse in the 3-s time window immediately after CSSU onset. ee, Quantification of the difference between neural and behavioral change-points, for all the neurons excited by CSQU and the corresponding mice in false alarm (FA) trials. ff, Histogram showing the distribution of neurons based on trial-by-trial Pearson correlation coefficients between their average activities and the licking rates of the mouse in the 3-s time window immediately after CSQU onset. Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Extended Data Fig. 2 Imaging Sst+ CeA neuron activities with or without learning.
a, Top and middle panels: trial-by-trial licking events for a representative mouse in response to a sound in naïve state (top), and in response to the sound (CSWA) and water (USWA) after training in the Pavlovian reward conditioning (middle). Bottom: average licking rate across trials for this mouse after training. b, Top and middle panels: trial-by-trial pupil size change for a representative mouse in response to a sound before training (top), and in response to the sound (CSSH) and shock (USSH) after training in the Pavlovian fear conditioning (middle). Bottom: average pupil size change across trials for this mouse after training. c, Quantification of the percentage of Sst+ CeA neurons showing inhibitory responses to different stimuli before and after training (n = 4 mice; CSWA, P = 0.8240, USWA, P = 0.3534, CSSH, P = 0.2754; n.s., nonsignificant, paired t-test). d, Pie charts showing the percentage distributions of Sst+ CeA neurons according to their response profiles to CS and US after training in reward conditioning (left) or fear conditioning (right). e, Footprints of Sst+ CeA neurons in a representative mouse imaged at naïve (left) stage and after training (right). After image alignment, the neurons detected in both sessions are labelled in green. f, Average activity traces of three example tracked neurons in different types of trials at naïve stage (upper two rows) and after training (bottom row). g, Left, the number of neurons detected at different training stages across mice. Right, the percentage of tracked neurons at different training stages across mice (n = 4 mice). h, Quantification of the percentage of tracked neurons among the CSWA- or USWA-responsive neurons in trained mice. Note that all the CSWA-responsive neurons were newly acquired during training; by contrast, 50% of the USWA-responsive neurons were newly acquired during training, and the other 50% of these neurons existed at the naïve stage. i, The population activities of tracked neurons in one representative mouse in response to CSWA and USWA at naïve stage (left) and after training (right). The first three principal components of the population activities are projected onto a 3D space. j, Quantification of the Mahalanobis distance between the vector representing CSWA responses and that representing USWA responses across training stages (n = 4 mice; *P = 0.0267, paired t-test). k–p, Tracking Sst+ CeA neuron activities across days without learning. k, Four example neurons showed stable responses to water, sucrose, food, or shock on day 1 and day 5. l, Percentage of responsive neurons to each stimulus across mice on day 1 and day 5 (n = 4 mice; water, P = 0.6376; sucrose, P = 0.6139; food, P = 0.5636; shock, *P = 0.0486; n.s., nonsignificant; paired t-test). m, Percentage distributions of neurons excited by different stimuli on day 1, day 3 and day 5 (Fisher’s exact test on the overlaps, water/sucrose: day 1, ****P = 4.28e-13, day 3, ****P < 1e-15, day 5, ****P = 4.09e-11; water/food: day 1, P = 0.3818, day 3, P = 0.1163, day 5, P = 0.1362; water/shock: day 1, P = 0.5178, day 3, P > 0.9999, day 5, P = 0.5527; sucrose/food: day 1, P = 0.2684, day 3, P = 0.2823, day 5, P = 0.8011; food/shock: day 1, P = 0.4434, day 3, P = 0.6465, day 5, P > 0.9999). n, Footprints of Sst+ CeA neurons in a representative mouse imaged on day 1 (left) and day 5 (right). After image alignment, the neurons detected in both sessions are labelled in green. o, Neurons were tracked across day 1 and day 5. Each Venn diagram shows the relationship between the neurons excited by a stimulus on day 1 and those excited by the same stimulus on day 5 (Fisher’s exact test on the overlaps: water, ****P = 2.32e-09; sucrose, ****P = 5.36e-06; food, **P = 0.0016; shock, **P = 0.0014). The percentage distributions are calculated based on all the tracked neurons (207 neurons in 4 mice). p, Each pie chart shows, for the neurons excited by a stimulus on day 1, the fractions of these neurons excited by other stimuli on day 5. Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Extended Data Fig. 3 Stimulus-specific responses in Sst+ CeA neurons during the retrieval of appetitive and aversive memories.
a, Average licking rate of a representative mouse in response to CSWA in the retrieval test. b, Average eye size change in a representative mouse in response to CSSH in the retrieval test. c, Two example Sst+ CeA neurons exhibiting different responses to CSWA and CSSH in the retrieval test. d, Pie charts showing the percentage distributions of Sst+ CeA neurons according to their response profiles to the CS in the retrieval test for the appetitive (left) or fear memory (right). e, Percentage distributions of the neurons excited by CSWA, CSSH, or both stimuli (overlap) in the retrieval test (Fisher’s exact test on the overlap: P = 0.1821). f, Left, an example of SVM decoding using the principal components (PC) of Sst+ CeA population activities in response to CSWA and CSSH in the retrieval test. Right, performance of the decoding as shown in the left. Actual, decoding analysis using the actual responses of neurons to CSWA and CSSH; shuffle, decoding analysis using the responses of neurons that were shuffled across trial types (n = 4 mice, **P = 0.0037, paired t-test). g, Trial-by-trial licking events for a representative mouse in response to CSWA during the retrieval test. h, Average licking rate in early trials was higher than late trials during retrieval of the appetitive memory (n = 4 mice, ***P = 0.0002, t-test). i, Top: trial-by-trial responses of two example neurons to CSWA. Bottom, average responses of the respective neurons on the top, for the first 5 and last 5 trials during the retrial test. j & k, Quantification of the CSWA responses for neurons showing higher (j) or lower response (k) to CSWA in the first 5 trials than the last 5 trials in the retrieval test (n = 15 neurons, ****P = 6.10e-05, Wilcoxon matched-pairs signed rank test; n = 8 neurons, **P = 0.003, paired t-test). l, Trial-by-trial blinking behavior for a representative mouse in response to CSSH during the retrieval test. m, Average eye size change in early trials was higher than late trials during retrieval of the fear memory (n = 4 mice, *P = 0.0219, t-test). n, Top: trial-by-trial responses of two example neurons to CSSH. Bottom, average responses of the respective neurons on the top, for the first 5 and last 5 trials during the retrial test. o & p, Quantification of the CSSH responses for neurons showing higher (o) or lower response (p) to CSSH in the first 5 trials than the last 5 trials in the retrieval test (n = 5 neurons, n.s., nonsignificant, P = 0.0823; n = 14 neurons, **P = 0.008, paired t-test). Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Extended Data Fig. 4 Imaging Sst+ CeA neuron activities in the go/no-go task.
a, A schematic of the go/no-go task design. b, Performance of mice (n = 4 mice) in the go/no-go task at the early and late training stages. c, Percentage of responsive and non-responsive neurons imaged at the early and late training stages (n = 4 mice). d, Heat-maps of the activities of all neurons in different trial types at the early and late training stages. Each row in each panel represents the activities of one neuron. Neurons in each panel are sorted based first on the response to CS and subsequently on the response to US. Neurons in CR trials are sorted in the same order as that in FA trials. e, Quantification of the fractions of neurons showing inhibitory responses in different trial types at different stages of training (n = 4 mice). f, Heat-maps of the activities of all tracked neurons in different trial types at the early and late training stages. Each row represents the activities of one neuron. All neurons are sorted based on the responses in the late training stage in Hit trials. Data in b, c, e are presented as mean ± s.e.m.
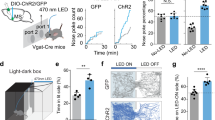
Extended Data Fig. 5 Control experiments for optogenetic manipulation.
a–g, Optogenetic inhibition of Sst+ CeA neurons during US presentation in well-trained mice does not impair performance. a, A representative ArchT mouse (left) and GFP mouse (right) used in Fig. 4 was given additional 8 sessions of training in the go/no-go task in the absence of laser stimulation. The licking events, sorted according to trial types, for the two mice in session 8 (S8) of the additional training, in which they have reached similar levels of performance and anticipatory licking. b, All the mice used in Fig. 4 were given additional 8 sessions of training in the go/no-go task in the absence of laser stimulation. Their performance and anticipatory licking rate in different sessions and trial types were quantified (ArchT group, n = 6 mice, GFP group, n = 5 mice; last session, n.s., nonsignificant, P > 0.05; t-test). c & d, The same mice as in b were tested in another 3 sessions, in which the laser stimulation was delivered to the CeA immediately following the onset of US delivery in each trial. c, Behavior of a representative ArchT mouse (left) and GFP mouse (right) in the 3rd of the test sessions. The licking events were sorted according to trial types. Dashed lines indicate the onset of CS and US. The green shaded area indicates the time window in a trial when the laser was turned on. d, Behavior of all the mice across sessions during the test. Their performance and anticipatory licking rate in different sessions and trial types were quantified (n.s., P > 0.05; two-way ANOVA). e, Confocal histological images of coronal brain sections from a representative mouse used for the experiments, showing ArchT-GFP expression in Sst+ CeA neurons and the locations of optical fiber implantation. f, Schematics showing the placement of fiber implants in the ArchT mice (n = 6 mice) used for the experiments. g, Confocal histological images of a coronal brain section from a representative mouse, showing ArchT-GFP expression (left) and the expression of Sst recognized by an antibody (middle). Almost all ArchT-GFP+ cells are also Sst+ (right). h–o, Optogenetic inhibition of Sst+ CeA neurons during CS presentation in well-trained mice does not impair performance. h & i, Behavior of well-trained ArchT mice in the reward-only task. h, The licking events of a representative mouse sorted according to laser and no-laser trials. Dashed lines indicate the onset of CS and US. The green shaded area (2 s) indicates the time window in a trial when the laser was turned on (50% of trials). i, The performance (left) and anticipatory licking rate (right) of all the mice in laser and no-laser trials (n = 7 mice, n.s., nonsignificant, P > 0.05; paired t-test). j & k, Behavior of the same mice in i after being well trained in the go/no-go task. j, The licking events of a representative mouse sorted according to laser and no-laser trials in the go trials. Dashed lines indicate the onset of CS and US. The green shaded area (2 s) indicates the time window in a trial when the laser was turned on (50% of go trials). k, The performance (left) and anticipatory licking rate (right) of all the mice in laser and no-laser trials (n.s., P > 0.05; paired t-test). l & m, Same as j & k, except that the no-go trials are used for the presentation (l) and analyses (m) (n.s., P > 0.05; paired t-test). n, Histological images of coronal brain sections from a representative mouse used for the experiments, showing ArchT-GFP expression in Sst+ CeA neurons and the locations of optical fiber implantation. o, Schematics showing the placement of fiber implants in the ArchT mice (n = 7 mice) used for the experiments. p–t, Optogenetic inhibition of Sst+ CeA neurons does not induce aversion or preference and has no effect on movements. p, Heat-maps for the activity of a representative ArchT mouse at baseline (top), or in a situation whereby entering the left (middle) or right (bottom) side of the chamber triggered photo-inhibition of Sst+ CeA neurons (i.e., the real-time place preference/aversion (RTPP/RTPA) test). q, Quantification of the behavior as shown in a, for mice in which Sst+ CeA neurons expressed ArchT (n = 6 mice, top) or GFP (n = 7 mice, bottom) (n.s., nonsignificant), P > 0.05, one-way ANOVA. r, Quantification of moving velocity (top) and distance (bottom) for the ArchT mice in q in the RTPP/RTPA test (n.s., P > 0.05, one-way ANOVA). s & t, Experiment with the continuous licking task. s, A schematic of the setup for the task. t, Quantification of the effect of laser stimulation on licking rate in ArchT mice and GFP mice (n.s., P > 0.05, one-way ANOVA). u–w, Optogenetic inhibition of SstCeA→DA projections in the go/no-go task after mice learned the reward task. The same mice used in Fig. 5b were further trained in the absence of laser stimulation such that the ArchT group and GFP group reached similar performance in the reward-only task. The two groups were subsequently trained in the go/no-go task, during which a green light (3 s) was delivered into the CeA immediately after the onset of US presentation in each trial throughout the training. u, Top left, hit rate across training sessions (ArchT group, n = 10 mice, GFP group, n = 11 mice; F(1,19) = 0.1965, n.s., nonsignificant, P = 0.6626; two-way ANOVA). Top right, licking rate following CS onset in go trials across training sessions (F(1,19) = 0.4038, n.s., P = 0.5327; two-way ANOVA). Bottom left, false alarm rate across training sessions (F(1,19) = 0.1985, n.s., P = 0.6610; two-way ANOVA). Bottom right, licking rate following CS onset in no-go trials across training sessions (F(1,19) = 0.7804, n.s., P = 0.388; two-way ANOVA). v, Histological images of coronal brain sections from a representative mouse used for the experiments, showing ArchT-GFP expression in Sst+ CeA neurons (top) and the locations of optical fiber implantation in the SNc (bottom). w, Schematics showing the placement of fiber implants in the SNc of the ArchT mice used for the experiment. Data are presented as mean ± s.e.m.
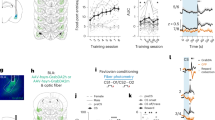
Extended Data Fig. 6 The SstCeA→DA projections.
a, A schematic of the approach. b, Images of the expression of the red fluorescent protein mRuby in Sst+ CeA neurons (left) and their projections to the SNc (right) in a representative SstCre mouse as prepared in a. c, A schematic of retrograde tracing with CTB injection into the SNc (left), and an image showing the injection in a representative SstCre;R26LSL-H2B-GFP mouse (right). d, Confocal images showing the CTB labelled neurons (left), Sst+ neurons (middle), and their overlap in the CeA (right). e, Quantification of the Sst+ neurons among CTB-labelled neurons in the CeA (n = 8 mice). f, g & h, Same as c, d & e, respectively, except that CTB was injected into the VTA (n = 5 mice). CeL, lateral subdivision of the CeA; CeM, medial subdivision of the CeA. i, A schematic of the approach for anterograde transsynaptic tracing of Sst+ CeA neurons. j, Confocal images showing CeA neurons infected by AAV-DIO-EGFP-T2A-TK (left) and HSV-ΔTK-tdTomato (middle), which are the two components of the anterograde transsynaptic tracing system. The “starter cells” are the neurons infected by both viruses (i.e., the yellow cells on the right). k, Confocal images showing the postsynaptic cells labelled by HSV-ΔTK-tdTomato (left panels), which are located in the SNc (top panels) and VTA (bottom panels). The DA neuronal marker tyrosine hydroxylase (TH) was recognized by an antibody (middle panels). Almost none of the HSV-labelled (tdTomato+) neurons expressed TH (right panels). l, Quantification of the non-TH cells (which are putative GABAergic neurons) among all the tdTomato+ neurons in the SNc and VTA (n = 5 mice). Data are presented as mean ± s.e.m.
Extended Data Fig. 7 Imaging SstCeA→DA neurons.
a, Schematics of the approach to label SstCeA→DA neurons with GCaMP6. b, A schematic of the approach for imaging. c, Confocal histological images of coronal brain sections from a representative mouse used for the experiment. Left: expression of GCaMP6 in SstCeA→DA neurons and the locations of GRIN Lens implantation. Middle and right: infection of neurons in the SNc (middle) and VTA (right) by the AAV8-retro-hsyn-mTagBFP-P2A-Cre, as indicated by the expression of BFP. d, Schematics showing the placement of GRIN lens implants in the mice (n = 4 mice) used for the experiment. e, Heat-maps of the responses of all neurons to different stimuli. Each row represents the activities of one neuron. f, The first three principle components (PC) (explain 82% of the variance) and hierarchical clustering dendrogram showing the relationship of each neuron within the three clusters. g, Quantification of the within-cluster sum of squares (WCSS) in f. h, Average responses of each cluster to different stimuli (n = 59 neurons/4 mice). i, Correlation coefficient matrix of the responses of all neurons for each stimulus pair. j, Percentage distributions of neurons excited by different stimuli (Fisher’s exact test on the overlaps: water/sucrose, ****P = 1.20e-06; water/shock, P > 0.9999; sucrose/shock, P = 0.5768). k, The responses of two example SstCeA→DA neurons to different stimuli. Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Extended Data Fig. 8 Activation of Sst+ CeA neurons promotes DA neuron activity.
a, A schematic of the approach to record SNc DA neuron activity while activating Sst+ CeA neurons. b, Top: trial-by-trial activity heat-maps of SNc DA neurons in one mouse, showing the responses induced by optogenetic activation of Sst+ CeA neurons. Bottom: trial-by-trial licking events from the same mouse. c, Top: calcium-dependent (green) and the simultaneously recorded isosbestic (black) GCaMP6 fluorescence signals in SNc DA neurons of the mouse in b. Bottom: average licking rate of the mouse. Blue bars represent laser stimulation (200 ms) in the CeA. d, Quantification of average neural activities (top) and licking rate (bottom) in 2-s time windows immediately before (“baseline”) and after (“response”) the laser stimulation (n = 5 mice; top, **P = 0.0066; bottom, n.s., nonsignificant, P = 0.1778; paired t-test). e, f & g, same as b, c & d, respectively, except that water was given to mice instead of optogenetic stimulation in the CeA (quantification in g: n = 5 mice; top, *P = 0.0121; bottom, ****P = 3.38e-05; paired t-test). h & i, SNc DA neuron activity in the GFP control mice. h, Left: calcium-dependent (green) and the simultaneously recorded isosbestic (black) GCaMP6 fluorescence signals in SNc DA neurons of a mouse. Right: quantification of average neural activities in 2-s time windows immediately before (“baseline”) and after (“response”) the laser stimulation (n = 5 mice, n.s., P = 0.3065; paired t-test). i, same as h, except that water was given to mice instead of optogenetic stimulation in the CeA (n = 5 mice, **P = 0.0046; paired t-test). j & k, same as h & i, respectively, except that VTA DA neuron activity was recorded. j, Quantification: n = 5 mice, n.s., P = 0.6973; paired t-test. k, Quantification: n = 5 mice, **P = 0.0079; Mann-Whitney test. l–o, Histology of the mice. l, Histological images of coronal brain sections from a representative mouse, showing ChR2-eYFP expression in Sst+ CeA neurons and the location of optical fiber implantation in the CeA (top), as well as GCaMP6 expression in SNc DA neurons and the location of optical fiber implantation in the SNc (bottom). m, Schematics showing the placement of fiber implants in the CeA for optogenetic stimulation (top), and in the SNc of the same mice for photometry (bottom) (n = 5 mice). n & o, same as l & m, respectively, except that photometry recording was performed in the VTA (n = 6 mice). Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Extended Data Fig. 9 DA neuron responses to appetitive stimuli are dependent on Sst+ CeA neurons.
a, Left: trial-by-trial activities of a water-excited neuron in a mouse receiving water, before (top) and after (bottom) the mouse was treated with DMSO. Right, trial-by-trial activities of another water-excited neuron in a mouse receiving water, before (top) and after (bottom) the mouse was treated with SALB. b, Quantification of the average water-evoked responses of all water-excited DA neurons in individual mice (n = 4 mice; DMSO, n.s., nonsignificant, P = 0.5807; SALB, *P = 0.0212; paired t-test). c & d, same as a & b, respectively, except that shock-excited neurons were analyzed, and mice received shock instead of water (quantification in d: n = 4 mice; DMSO, n.s., P = 0.0794; SALB, n.s., P = 0.3593; paired t-test). e, Left: trial-by-trial (top and middle) and average (bottom) activities of a sucrose-excited neuron in a mouse receiving sucrose, before and after the mouse was treated with DMSO. Right: trial-by-trial (top and middle) and average (bottom) activities of another sucrose-excited neuron in a mouse receiving sucrose, before and after the mouse was treated with SALB. f, Top: Quantification of sucrose-induced responses in DA neurons in one example mouse. All DA neurons showing excitatory responses to sucrose were included (DMSO, n = 16 neurons, n.s., P = 0.8982; SALB, n = 25 neurons, **P = 0.0019; paired t-test). Bottom: quantification of the average sucrose-evoked responses of all sucrose-excited DA neurons in individual mice (n = 4 mice; DMSO, n.s., P = 0.9505; SALB, *P = 0.0132; paired t-test). g & h, same as e & f, respectively, except that quinine-excited neurons were analyzed, and mice received quinine instead of sucrose (quantification in h, top: DMSO, n = 14 neurons, n.s., P = 0.5657; SALB, n = 15 neurons, n.s., P = 0.0984; paired t-test; bottom: n = 4 mice; DMSO, n.s., P = 0.4903; SALB, n.s., P = 0.4020; paired t-test). i & j, same as g & h, respectively, except that quinine-inhibited neurons were analyzed (quantification in j, top: DMSO, n = 3 neurons, n.s., P = 0.7500; SALB, n = 3 neurons, n.s., P = 0.7500; Wilcoxon test; bottom: n = 4 mice; DMSO, n.s., P = 0.5906; SALB, n.s., P = 0.9527; paired t-test). k & l, same as i & j, respectively, except that shock-inhibited neurons were analyzed, and mice received shock instead of quinine (quantification in l, top: DMSO, n = 4 neurons, n.s., P = 0.6961; SALB, n = 4 neurons, n.s., P = 0.3916; paired t-test; bottom: n = 4 mice; DMSO, n.s., P = 0.8263; SALB, n.s., P = 0.2366; paired t-test). Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Extended Data Fig. 10 Assessing the effects of Sst+ CeA neuron inhibition on DA neuron responses to expected and unexpected reward.
a, A schematic of the task. After mice were trained in the reward conditioning task where a sound predicted the delivery of water reward, they underwent an imaging session during which expected reward and unexpected reward were delivered in randomly interleaved trials. N/A, not applicable. b, Top and middle: raster plots of licking events for a mouse in unexpected-reward trials and expected-reward trials sorted according to trial types. The mouse was treated with DMSO (left) or SALB (right). Bottom: average licking rate of this mouse in different types of trials. Dashed lines indicate the onset of CS and US. c, Quantification of licking rate in a 2-s time window immediately after CS or US presentation in different conditions (n = 3 mice; DMSO: CS, P = 0.7133; expected US, P > 0.9999; unexpected US, P = 0.7745; SALB: CS, P = 0.0572; expected US, P = 0.1317; unexpected US, P = 0.5799; n.s., nonsignificant; paired t-test). d, Percentage distributions of neurons excited by CS, expected reward, or unexpected reward, in mice treated with DMSO (left) or SALB (right). Fisher’s exact test on the overlaps, DMSO: CS / expected reward, *P = 0.0121; CS / unexpected reward, **P = 0.0076; expected reward / unexpected reward, *P = 0.0119; SALB: CS / expected reward, ***P = 0.0005; CS / unexpected reward, P = 0.0680; expected reward / unexpected reward, ****P = 6.68e-07. e, Left and middle panels: heat-maps of trial-by-trial responses of a PE-encoding DA neuron in expected-reward trials (left) and unexpected-reward trials (middle), before (top) and after (bottom) the mouse was treated with DMSO. Right panel: average activity traces of this neuron in different trial types, before (top) and after (bottom) the mouse was treated with DMSO. f, same as e, except that SALB was used instead of DMSO to treat the mouse. g-i, Histology of the mice in which Sst+ CeA neurons were chemogenetically inhibited and VTA DA neuron activity was imaged through a GRIN Lens. g, A confocal histological image of a coronal brain section from a representative mouse, showing the infection of Sst+ CeA neurons with an AAV expressing KORD. h, Confocal histological images of a coronal brain section from a representative mouse, showing the infection of VTA DA neurons with an AAV expressing GCaMP6 (left), TH expression in DA neurons recognized with an antibody (middle). GCaMP6 expression was restricted in TH+ neurons in the VTA (right). The track of GRIN lens implantation was indicated. i, Schematics showing the placement of GRIN lens implants in the VTA (n = 8 mice) used for the experiments. Note that some mice were used in more than one experiment, as indicated. Data are presented as mean ± s.e.m. Shaded areas represent s.e.m.
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, T., Yu, K., Zhang, X. et al. Plastic and stimulus-specific coding of salient events in the central amygdala. Nature 616, 510–519 (2023). https://doi.org/10.1038/s41586-023-05910-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05910-2
This article is cited by
-
Intermediate-term memory mechanism inspired lightweight single image super resolution
Multimedia Tools and Applications (2024)
-
Abnormalities of the Amygdala in schizophrenia: a real world study
BMC Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.