Abstract
Haematopoietic stem cells (HSCs) are a rare cell type that reconstitute the entire blood and immune systems after transplantation and can be used as a curative cell therapy for a variety of haematological diseases1,2. However, the low number of HSCs in the body makes both biological analyses and clinical application difficult, and the limited extent to which human HSCs can be expanded ex vivo remains a substantial barrier to the wider and safer therapeutic use of HSC transplantation3. Although various reagents have been tested in attempts to stimulate the expansion of human HSCs, cytokines have long been thought to be essential for supporting HSCs ex vivo4. Here we report the establishment of a culture system that allows the long-term ex vivo expansion of human HSCs, achieved through the complete replacement of exogenous cytokines and albumin with chemical agonists and a caprolactam-based polymer. A phosphoinositide 3-kinase activator, in combination with a thrombopoietin-receptor agonist and the pyrimidoindole derivative UM171, were sufficient to stimulate the expansion of umbilical cord blood HSCs that are capable of serial engraftment in xenotransplantation assays. Ex vivo HSC expansion was further supported by split-clone transplantation assays and single-cell RNA-sequencing analysis. Our chemically defined expansion culture system will help to advance clinical HSC therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Exome-sequencing data are available on BioProject (PRJNA786760). All RNA-seq data have been deposited in the GEO under the accession codes GSE191338 (bulk) and GSE192519 (single cell). Source data are provided with this paper.
References
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354, 1813–1826 (2006).
Cohen, S. et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 7, e134–e145 (2020).
Pineault, N. & Abu-Khader, A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp. Hematol. 43, 498–513 (2015).
Wilkinson, A. C. & Nakauchi, H. Stabilizing hematopoietic stem cells in vitro. Current Opin. Genet. Dev. 64, 1–5 (2020).
Gluckman, E. et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N. Engl. J. Med. 321, 1174–1178 (1989).
Orkin, S. H. & Zon, L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008).
Weissman, I. L. Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168 (2000).
Wilkinson, A. C., Igarashi, K. J. & Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 21, 541–554 (2020).
Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 (2010).
Fares, I. et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345, 1509–1512 (2014).
Wagner, J. E. Jr. et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell 18, 144–155 (2016).
Bai, T. et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 25, 1566–1575 (2019).
Grey, W. et al. Activation of the receptor tyrosine kinase RET improves long-term hematopoietic stem cell outgrowth and potency. Blood 136, 2535–2547 (2020).
Huang, J., Nguyen-McCarty, M., Hexner, E. O., Danet-Desnoyers, G. & Klein, P. S. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat. Med. 18, 1778–1785 (2012).
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019).
Wilkinson, A. C., Ishida, R., Nakauchi, H. & Yamazaki, S. Long-term ex vivo expansion of mouse hematopoietic stem cells. Nat. Protoc. 15, 628–648 (2020).
Ieyasu, A. et al. An all-recombinant protein-based culture system specifically identifies hematopoietic stem cell maintenance factors. Stem Cell Rep. 8, 500–508 (2017).
Seita, J. et al. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc. Natl Acad. Sci. USA 104, 2349–2354 (2007).
Park, H. J. et al. Cytokine-induced megakaryocytic differentiation is regulated by genome-wide loss of a uSTAT transcriptional program. EMBO J. 35, 580–594 (2016).
Yamazaki, S. et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 25, 3515–3523 (2006).
Miyamoto, K. et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1, 101–112 (2007).
Tadokoro, Y. et al. Spred1 safeguards hematopoietic homeostasis against diet-induced systemic stress. Cell Stem Cell 22, 713–725 (2018).
Lechman, E. R. et al. Attenuation of miR-126 activity expands HSC in vivo without exhaustion. Cell Stem Cell 11, 799–811 (2012).
Sakurai, M., Takemoto, H., Mori, T., Okamoto, S. & Yamazaki, S. In vivo expansion of functional human hematopoietic stem progenitor cells by butyzamide. Int. J. Hematol. 111, 739–741 (2020).
Nishimura, T. et al. Use of polyvinyl alcohol for chimeric antigen receptor T-cell expansion. Exp. Hematol. 80, 16–20 (2019).
Ito, M. et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 (2002).
Linn, M. et al. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur. J. Pharm. Sci. 45, 336–343 (2012).
Jin, X., Zhou, B., Xue, L. & San, W. Soluplus(®) micelles as a potential drug delivery system for reversal of resistant tumor. Biomed. Pharmacother. 69, 388–395 (2015).
Sudo, K., Yamazaki, S., Wilkinson, A. C., Nakauchi, H. & Nakamura, Y. Polyvinyl alcohol hydrolysis rate and molecular weight influence human and murine HSC activity ex vivo. Stem Cell Res. 56, 102531 (2021).
Ito, R. et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J. Immunol. 191, 2890–2899 (2013).
Fares, I. et al. EPCR expression marks UM171-expanded CD34+ cord blood stem cells. Blood 129, 3344–3351 (2017).
Lehnertz, B. et al. HLF expression defines the human hematopoietic stem cell state. Blood 138, 2642–2654 (2021).
Aguilo, F. et al. Prdm16 is a physiologic regulator of hematopoietic stem cells. Blood 117, 5057–5066 (2011).
Che, J. L. C. et al. Identification and characterization of in vitro expanded hematopoietic stem cells. EMBO Rep. 23, e55502 (2022).
García-Prat, L. et al. TFEB-mediated endolysosomal activity controls human hematopoietic stem cell fate. Cell Stem Cell 28, 1838–1850 (2021).
Liang, R. et al. Restraining lysosomal activity preserves hematopoietic stem cell quiescence and potency. Cell Stem Cell 26, 359–376 (2020).
Lee-Six, H. et al. Population dynamics of normal human blood inferred from somatic mutations. Nature 561, 473–478 (2018).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Nocka, K. et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 9, 1805–1813 (1990).
Ema, H. et al. Adult mouse hematopoietic stem cells: purification and single-cell assays. Nat. Protoc. 1, 2979–2987 (2006).
Nogami, W. et al. The effect of a novel, small non-peptidyl molecule butyzamide on human thrombopoietin receptor and megakaryopoiesis. Haematologica 93, 1495–1504 (2008).
Sakurai, M., Ishitsuka, K. & Yamazaki, S. Cytokine-free ex vivo expansion of human hematopoietic stem cells. Protoc. Exch. (in the press).
Kuchimaru, T. et al. A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries. Nat. Commun. 9, 2981 (2018).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Nestorowa, S. et al. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood 128, e20–e31 (2016).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Acknowledgements
We thank M. Watanabe, Y. Yamazaki, Y. Ishii, M. Hayashi, R. Hirochika, M. Kikuchi and the Organization for Open Facility Initiatives, University of Tsukuba for technical support, and Y. Niitsu, M. Kawakatsu, T. Kajiura, K. Kolter and F. Guth for providing polymers. This research was funded by JSPS KAKENHI Grants-in-Aid for Scientific Research (JP20H03707, JP20H05025 and JP20K17407) and the Japan Agency for Medical Research and Development (AMED) (21bm0404077h0001 and 21bm0704055h0002). M.S. is supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (JP20K17407), Japanese Society of Hematology research grants (19056 and 20128) and a Nippon Shinyaku research grant. A.C.W. is supported by the Kay Kendall Leukaemia Fund, the NIHR, the Leukemia and Lymphoma Society (3385-19), the NIH (K99HL150218) and the UK National Institute of Medical Research. H.J.B. is supported by the German Research Foundation (BE 6847/1-1). The D.G.K lab is supported by an MRC-AMED Strategic Award in Stem Cells and Regenerative Medicine (MR/V005502/1). H. Nakauchi is supported by the California Institute for Regenerative Medicine (grant LA1_C12-06917), the US NIH (grants R01DK116944, R01HL147124 and R21AG061487), a JSPS KAKENHI Grant-in-Aid for Scientific Research (JSPS20181706) and the Virginia and D.K. Ludwig Fund for Cancer Research.
Author information
Authors and Affiliations
Contributions
M.S., K.I. and S.Y. conceived, designed and performed experiments, analysed data and wrote the paper. R.I., T.K., E.M., H. Nishikii, K.S., H.J.B. and H.T. designed and performed experiments. M.S. performed cell cultures, CFU assays, FACS analysis and xenotransplantation assays. K.I. performed cell cultures, FACS analysis, exome sequencing, RNA-seq, cell-cycle analysis, apoptosis assays, ROS assays and γH2AX assays. S.Y. performed cell cultures, signalling analysis, xenotransplantation assays and clonal HSC expansion assays. R.I. performed xenotransplantation assays. T.K. performed exome sequencing and RNA-seq. E.M., H. Nishikii, K.S., H.J.B. and H.T. helped with cell cultures and FACS analysis. S.Y. performed independent replications of the experiments (Supplementary Tables 1–4) in the University of Tokyo and University of Tsukuba, and R.I. performed independent replications of the experiments (Supplementary Tables 1 and 2) in the Central Institute for Experimental Animals. A.C.W. and D.G.K. analysed data and wrote the paper. T.S. provided reagents and discussed the results. K.K., S.T., Y.N., A.I., S.C. and S.O. discussed the results and wrote the paper. H. Nakauchi guided and supervised the project. All authors edited and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
M.S. and S.Y. are co-founders and shareholders in Celaid Therapeutics. H. Nakauchi is a co-founder and shareholder in Megakaryon, Century Therapeutics and Celaid Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks John Dick, Stephanie Halene, Ross Levine, Toma Tebaldi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
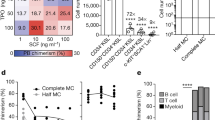
Extended Data Fig. 1 Development of a chemically defined cytokine-free culture medium for human HSPCs.
(a) Single-cell phosphorylation status of JAK2, STAT3, STAT5, p38 MAPK, and p44/42 MAPK in mouse KSL and human cord blood CD34+CD38− cells cultured with 10 ng/ml SCF and 100 ng/ml THPO in PVA-based medium. Mean of 30 cells. AFI: average fluorescence intensity. ****P < 0.0001. (b) Representative image of p-PI3K after 7 days culture cells in mouse and human, as described in (a). Blue: DAPI, Green: anti-PI3K. Scale bar: 100 µm. (c) Single-cell phosphorylation status of PI3K in mouse CD34−KSL and human CD34+CD38−CD90+CD45RA−CD49f+ cord blood cells cultured with 10 ng/ml SCF and 100 ng/ml THPO in PVA-based medium for 3 and 7 days. Mean of 31 cells. AFI: average fluorescence intensity. ****P < 0.0001. (d) CD34+CD45RA− cell numbers of human cord-blood-derived CD34+CD38− cultured with SC79 or 740Y-P in addition to human 10 ng/ml SCF (S) and 100 ng/ml THPO (T) in PVA culture conditions for 7 days. The starting cell count was 2x104. Mean of three independent cultures. **P = 0.0040. (e) Single-cell phosphorylation status of PI3K in human CD34+CD38−CD90+CD45RA−CD49f+ cord blood cells cultured in PVA-based medium containing 10 ng/ml SCF and 100 ng/ml THPO with or without 740Y-P for 7 days. Mean of 31 cells. AFI: average fluorescence intensity. ***P = 0.0002. (f) Cell-cycle analysis of CD34+CD45RA− cells after a 7-day culture of human cord blood CD34+ cells in PVA-based medium containing 740Y-P and 100 ng/ml THPO (T) with or without 10 ng/ml SCF (S). Mean of three independent cultures. Representative FACS plot was shown on the right. (g) Fold change in GEmM colony numbers generated from human cord blood CD34+ cells after a 7-day culture in PVA-based medium supplemented with 740Y-P and THPO (T) with or without SCF (S), relative to fresh CD34+ cells. Mean of three independent cultures. (h) Total cell numbers after 1x103 MPL-expressing 32D cells (32D/MPL) were cultured for 3-days with various THPO agonists (eltrombopag, avatrombopag or butyzamide) in BSA-based or PVA-based medium. Mean of two independent cultures. n.d., not detected. (i) Fold change in total and CD34+ cell numbers after a 7-day culture of 2x104 CD34+ cells in PVA-based medium supplemented with 740Y-P and various THPO agonists (eltrombopag, avatrombopag or butyzamide). Mean of three independent cultures. n.d., not detected. (j) CD34+CD41−CD90+CD45RA− cell numbers after a 7-day culture of 2x104 of human cord blood CD34+ cells in PVA-based medium containing 740Y-P and 100 ng/ml THPO (T) and/or butyzamide (Buty). Mean of three independent cultures. **P = 0.0020; ***P = 0.0010. (k) Fold change in GEmM colony numbers generated from CD34+ cells after a 7-day culture in PVA-based medium supplemented with 740Y-P and THPO (T) or butyzamide (Buty), relative to fresh CD34+ cells. Mean + S.D. of three independent cultures. **P = 0.0017. (l) The frequency of cells during the culture of 2x104 human cord blood CD34+ cells in PVA-based medium containing 1 µM 740Y-P and 0.1 µM butyzamide. Mean + S.D. of three independent cultures. (m) Representative image of a day-14 PVA-based culture containing 1 µM 740Y-P and 0.1 µM butyzamide (2a medium). Representative of at least five experiments. Scale bar: 100 µm. (n) MgK colony numbers obtained from 50 CD34+ cells sorted from day-7 and day-14 PVA-based cultures containing 1 µM 740Y-P and 0.1 µM butyzamide (2a medium). Mean + S.D. of three independent cultures. *P = 0.0161. (o, p) Mean human CD45+ peripheral blood (o) and bone marrow (p) chimerism in recipient NOD/Shi-scid IL-2Rγnull (NOG) mice following transplantation of 1x104 cells derived from a 7-day or 14-day culture of 1x104 cord blood CD34+ cells in PVA-based 2a medium containing 1 µM 740Y-P and 0.1 µM butyzamide. n = 5 mice per group. (o) **†P = 0.0036; **‡P = 0.0037; ***P = 0.0007, (p) ***P = 0.0003. Statistical significance was calculated using an unpaired two-tailed t-test. n.s., not significant.
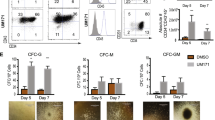
Extended Data Fig. 2 Long-term ex vivo expansion of human HSPCs in chemically defined cytokine-free cultures.
(a) CD34+ EPCR+ cell numbers after a 7-day culture of 2x104 human cord blood CD34+ cells in PVA-based medium containing 750 nM SR-1 and/or 70 nM UM171 in addition to 2a medium. Mean of three independent cultures. ****P < 0.0001. (b) CD34+ EPCR+ and CD34+EPCR+CD90+CD45RA−ITGA3+ cell numbers after a 14-day culture of 2x104 cord blood CD34+ cells in PVA-based 2a medium with or without 70 nM UM171. Mean of three independent cultures. ****P < 0.0001. (c) The frequency of CD34+EPCR+CD90+CD45RA−ITGA3+ cells after a 10-day culture of 2x104 human cord blood CD34+ cells in StemSpan SFEM supplemented with cytokines with 35 nM UM171 and PVA-based 2a medium with 35 or 70 nM UM171. Mean of three independent cultures. ****P < 0.0001. (d) Annexin V staining assay of total cells after a 7-day culture of 2x104 human cord blood CD34+ cells in PVA-based medium containing 750 nM SR-1 and/or 0.1% BSA in addition to 2a or 10 ng/ml SCF (S) and 100 ng/ml THPO (T). Mean of three independent cultures. ****P < 0.0001. (e) Mean human CD45+ peripheral blood chimerism in recipient NOG mice at 24 weeks following transplantation of 1x104 fresh cord blood CD34+ cells or the cells derived from a 10-day or 30-day culture of 1x104 cord blood CD34+ cells in PVA-based 3a medium. n=3 mice per group. Details described in Fig. 2d. *†P = 0.0495; *‡P = 0.0319. (f) Mean 24-week human CD45+, CD34+, CD34+CD38−CD90+CD45RA−CD49f+ cell chimerism in the bone marrow from mice described in (e). n = 3 mice per group. Details described in Fig. 2d. *†P = 0.0112; *‡P = 0.0480; *§P = 0.0187; **P = 0.0075; ***P = 0.0003; ****P < 0.0001. (g) Mean 24-week human CD45+, CD34+, CD34+CD38−CD90+CD45RA−CD49f+ cell chimerism in the spleen from mice described in (e). n = 3 mice per group. Details described in Fig. 2d. **P = 0.0014. Statistical significance was calculated using one-way ANOVA or an unpaired two-tailed t-test. n.s., not significant.
Extended Data Fig. 3 Caprolactam-polymer-based 3a medium supports the efficient expansion of human HSCs ex vivo.
(a) CD34+CD45RA− cell numbers of human cord-blood-derived CD34+ cultured with SC79 or 740Y-P in addition to human 10 ng/ml SCF (S) and 100 ng/ml THPO (T) in PCL-PVAc-PEG culture conditions for 7 days. The starting cell count was 2x104. Mean of three independent cultures. *P = 0.0210. (b) CD34+CD41−CD90+CD45RA− cell numbers after a 7-day culture of 2x104 human cord blood CD34+ cells in PCL-PVAc-PEG-based medium containing 740Y-P and 100 ng/ml THPO (T) and/or butyzamide (Buty). Mean of three independent cultures. *P = 0.0256. (c) CD34+EPCR+ and CD34+EPCR+CD90+CD45RA− cell numbers after a 7-day culture of 2x104 human cord blood CD34+ cells in PCL-PVAc-PEG -based 2a medium containing 750 nM SR-1 and/or 70 nM UM171 . Mean of three independent cultures. **P = 0.0021; ***P = 0.0002. (d) CD34+CD41−CD90+CD45RA− cell numbers after a 7-day culture of 2x104 of human cord blood CD34+ cells in PCL-PVAc-PEG-based medium containing 0-20 µM, 740Y-P, and 0.1 µM butyzamide. Mean of three independent cultures. *†P = 0.0214; *‡P = 0.0440. (e) The frequency of CD34+EPCR+CD90+CD45RA−ITGA3+ cells after a 7-day culture of 2x104 human cord blood CD34+ cells in PCL-PVAc-PEG-based 2a medium with 35 nM or 70 nM UM171. Mean of three independent cultures. (f) GEmM colony numbers generated from CD34+ cells after a 10-day culture in PVA- and/or PCL-PVAc-PEG-based 3a medium. Mean of three independent cultures. ***P = 0.0005. (g) Annexin V staining assay of total cells after a 7-day culture of 2x104 human cord blood CD34+ cells in PVA- or PCL-PVAc-PEG-based 2a medium containing 750 nM SR-1. Mean of three independent cultures. **P = 0.0086. (h) The frequency of propidium iodide (PI)-positive cells after a 7-day culture of 2x104 human cord blood CD34+ cells in PCL-PVAc-PEG-based 3a medium with or without 10 µM LY294002 (Chemscene, CAS No. 154447-36-6), PI3-kinase inhibitor. Mean of three independent cultures. ****P < 0.0001. (i) Total and CD34+EPCR+CD90+CD45RA−ITGA3+ cell numbers after a 10-day culture of 2x104 adult-PBSC CD34+ cells in PVA- or PCL-PVAc-PEG-based 3a medium including UM729 instead of UM171. Mean of three independent cultures. *P = 0.0153, **P = 0.0079. (j) Mean human CD45+ peripheral blood chimerism in recipient NOG mice at 24 weeks after transplantation of 1x104 day-30 cells derived from cord blood CD34+ cells cultured in 3a medium containing PVA or PCL-PVAc-PEG. n = 3 mice per group. Detailed described in Fig. 3c. (k) Mean 24-week human CD45+, CD34+, CD34+CD38−CD90+CD45RA−CD49f+ cell chimerism in the bone marrow from mice described in (j). n = 3 mice per group. Detailed described in Fig. 3c. (l) Mean 24-week human CD45+, CD34+, CD34+CD38−CD90+CD45RA−CD49f+ cell chimerism in the spleen from mice described in (j). n = 3 mice per group. Detailed described in Fig. 3c. Statistical significance was calculated using one-way ANOVA or an unpaired two-tailed t-test: n.s., not significant.
Extended Data Fig. 4 Comparison of human HSC culture protocols.
(a) Total cell numbers generated from a 10-day culture of 2x104 human cord blood CD34+ cells in PCL-PVAc-PEG or StemSpan SFEM-based cytokine cocktail medium with UM171 and/or SR-1 (see in Methods for details), or PCL-PVAc-PEG-based 3a medium. Mean of three independent cultures. ***P = 0.0002; ****P <0.0001. (b) The frequency and (c) absolute number of CD34+EPCR+CD90+CD45RA−ITGA3+ cells in cultures described in (a). Mean of three independent cultures. (b) ****P <0.0001. (c) **P = 0.0010; ***†P = 0.0009; ***‡P = 0.0002; ****P <0.0001. (d) The ROS and γH2AX level of fresh cord blood CD34+ cells (fresh) and CD34+ cells in cultures described in (a). Mean of three independent cultures. **P = 0.0028; ***P = 0.0004; ****P <0.0001. (e) Relative mean fluorescence intensity (MFI) for ROS and yH2AX in fresh cord blood CD34+ cells (fresh) and cells from 10- or 30-day PCL-PVAc-PEG-based 3a-medium cultures. Mean of three independent cultures. (f,g) Mean human CD45+ peripheral blood and bone marrow chimerism in recipient NOG mice following transplantation of 1x104 day-10 cells derived cultures as describe in (a). n = 4 mice per group. ***P = 0.0001; ****P <0.0001. (h,i) Mean human CD45+ peripheral blood and bone marrow chimerism in secondary recipient NOG mice following transplantation of 1x106 bone marrow cells derived from primary recipient mice, as described in (f,g). n = 3 mice per group. *†P = 0.0180; *‡P = 0.0299. Statistical significance was calculated using one-way ANOVA: n.s., not significant.
Extended Data Fig. 5 Gating strategy for HSC fractions by flow cytometry.
(a) FACS gating strategy for detecting CD34+EPCR+CD90+CD45RA−ITGA3+ cells after 10-day culture in 3a medium containing PCL-PVAc-PEG or using UM171/SR-1.
Extended Data Fig. 6 Profile of human HSCs expanded in 3a medium.
(a) Volcano plot showing differentially expressed genes (DEGs) detected in bulk RNA-seq of CD34highEPCR+ (right) and CD34highEPCR− (left) cells after 10-day culture in PCL-PVAc-PEG-based 3a medium. DEGs are highlighted as red dots (log2FC >2, -logP Value <14). Gene names are shown in the boxes. (b) GO Term cellular component-specific GSEA analysis performed on DEGs, displayed as a dot plot. (c) Expression of key genes within annotated clusters, displayed as a dot plot. (d,e) Feature plots showing HLF (c) and AVP (d) gene expression within the integrated cell map. (f) Ratio of each cell cluster within PCL-PVAc-PEG-based 3a cultures, StemSpan with SR-1 cultures, and StemSpan with UM171 cultures, as described in Fig. 4e. (g) Comparison of single-cell RNA-seq data from cells cultured for 10 days in PCL-PVAc-PEG-based 3a medium with two dataset of cells cultured for 7 days in StemSpan SFEM with UM171 cultures obtained from GEO (GSE153370). (h) Violin plots displaying HLF expression in cells from a 10-day culture using 3a medium, cells from UM171/SR-1 cultures, and two fresh cord blood cells from publicly available data (GSE153370).
Extended Data Fig. 7 Split-clone assays.
(a) Schematic of assay of the single HSC expansion and split-clone assay. Single human CD34+CD38−CD90+CD45RA−CD49f+ cord blood cells were sorted into 96 wells and expanded in 3a medium containing PCL-PVAc-PEG for 7 days. Individual HSC clones were then transplanted into three recipient W41/W41 mice. (b) Human CD45+ peripheral blood chimerism in recipient W41/W41 mice 24 weeks after transplantation of day-7 cells derived from single human CD34+CD38−CD90+CD45RA−CD49f+ cord blood cell cultured in 3a medium containing PCL-PVAc-PEG (3 mice/well), as described in (a).
Extended Data Fig. 8 NOG-W41/W41 mice exhibit high human haematopoietic cell chimerism.
(a) Mean human CD45+ peripheral blood chimerism in recipient NOD.Cg-Prkdcscid Il2rgtm1Sug Kitem1(V831M)Jic/Jic (W41/W41), W41/+, +/+ mice at 4, 8, 12, 16 and 20 weeks following transplantation of 5x104 fresh cord blood CD34+ cells. n = 3-4 mice per group. ***P = 0.0004; ****P <0.0001. (b) Mean 24-week human CD45+ cell chimerism in the bone marrow and spleen from mice described in (a). n = 3-4 mice per group. **†P = 0.0016; **‡P = 0.0021; ***†P = 0.0001; ***‡P = 0.0006. (c) Mean human CD45+, CD19+, CD33+, CD3+, CD56+ and CD66b+ peripheral blood chimerism in recipient non-irradiated or irradiated (0.5 Gy) W41/W41 mice at 4-, 8-, 12- and 16 weeks following transplantation of 5x104 fresh cord blood CD34+ cells. Mean + S.D of 3-4 mice per group. *†P = 0.0257; *‡P = 0.0335; **†P = 0.0030; **‡P = 0.0060; ***P = 0.0002. Statistical significance was calculated using one-way ANOVA or an unpaired two-tailed t-test: n.s., not significant.
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakurai, M., Ishitsuka, K., Ito, R. et al. Chemically defined cytokine-free expansion of human haematopoietic stem cells. Nature 615, 127–133 (2023). https://doi.org/10.1038/s41586-023-05739-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05739-9
This article is cited by
-
Generating human bone marrow organoids for disease modeling and drug discovery
Nature Protocols (2024)
-
Umbilical cord blood and cord tissue banking as somatic stem cell resources to support medical cell modalities
Inflammation and Regeneration (2023)
-
Long-term expansion of human hematopoietic stem cells
Cell Regeneration (2023)
-
Towards clinically meaningful expansion of human HSCs
Cell Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.