Abstract
Ribosomes are produced in large quantities during oogenesis and are stored in the egg. However, the egg and early embryo are translationally repressed1,2,3,4. Here, using mass spectrometry and cryo-electron microscopy analyses of ribosomes isolated from zebrafish (Danio rerio) and Xenopus laevis eggs and embryos, we provide molecular evidence that ribosomes transition from a dormant state to an active state during the first hours of embryogenesis. Dormant ribosomes are associated with four conserved factors that form two modules, consisting of Habp4–eEF2 and death associated protein 1b (Dap1b) or Dap in complex with eIF5a. Both modules occupy functionally important sites and act together to stabilize ribosomes and repress translation. Dap1b (also known as Dapl1 in mammals) is a newly discovered translational inhibitor that stably inserts into the polypeptide exit tunnel. Addition of recombinant zebrafish Dap1b protein is sufficient to block translation and reconstitute the dormant egg ribosome state in a mammalian translation extract in vitro. Thus, a developmentally programmed, conserved ribosome state has a key role in ribosome storage and translational repression in the egg.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM maps and molecular models generated in this study have been deposited in the Protein Data Bank (PDB) under accessions 7OYA (zebrafish 1 hpf), 7OYB (zebrafish 6 hpf), 7OYC (Xenopus eggs) and 7OYD (rabbit ribosome with zebrafish Dap1b), and in the Electron Microscopy Data Bank with accession codes EMD-13111 (zebrafish 1 hpf), EMD-13112 (zebrafish 6 hpf), EMD-13113 (Xenopus egg ribosome) and EMD-13114 (rabbit ribosome with zebrafish Dap1b). Raw micrographs, particle stacks and CryoDRGN models were deposited at the EMPIAR Database (EMPIAR-11274). Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE83 partner repository with the dataset identifier PXD026866. Source data are provided with this paper.
Code availability
No custom codes were used for this study.
References
Woodland, H. R. Changes in the polysome content of developing Xenopus laevis embryos. Dev. Biol. 40, 90–101 (1974).
Brandis, J. W. & Raff, R. A. Translation of oogenetic mRNA in sea urchin eggs and early embryos. Demonstration of a change in translational efficiency following fertilization. Dev. Biol. 67, 99–113 (1978).
Kronja, I. et al. Widespread changes in the posttranscriptional landscape at the Drosophila oocyte-to-embryo transition. Cell Rep. 7, 1495–1508 (2014).
Bachvarova, R. & De Leon, V. Stored and polysomal ribosomes of mouse ova. Dev. Biol. 58, 248–254 (1977).
Burkholder, G. D., Comings, D. E. & Okada, T. A. A storage form of ribosomes in mouse oocytes. Exp. Cell. Res. 69, 361–371 (1971).
Alberts, B. et al. in Molecular Biology of the Cell 5th edn (eds Anderson, M. & Granum, S.) 1287–1291 (Garland Science, 2008).
Locati, M. D. et al. Linking maternal and somatic 5S rRNA types with different sequence-specific non-LTR retrotransposons. RNA 23, 446–456 (2017).
Locati, M. D. et al. Expression of distinct maternal and somatic 5.8S, 18S, and 28S rRNA types during zebrafish development. RNA 23, 1188–1199 (2017).
Cenik, E. S. et al. Maternal ribosomes are sufficient for tissue diversification during embryonic development in C. elegans. Dev. Cell 48, 811–826.e6 (2019).
Danilchik, M. V. & Hille, M. B. Sea urchin egg and embryo ribosomes: differences in translational activity in a cell-free system. Dev. Biol. 84, 291–298 (1981).
Chassé, H., Boulben, S., Cormier, P. & Morales, J. Translational control of canonical and non-canonical translation initiation factors at the sea urchin egg to embryo transition. Int. J. Mol. Sci. 20, 626 (2019).
Subtelny, A. O., Eichhorn, S. W., Chen, G. R., Sive, H. & Bartel, D. P. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 508, 66–71 (2014).
Stebbins-Boaz, B., Cao, Q., Moor, C. H., de, Mendez, R. & Richter, J. D. Maskin is a CPEB-associated factor that transiently interacts with eIF-4E. Mol. Cell 4, 1017–1027 (1999).
Smith, P. R., Pandit, S. C., Loerch, S. & Campbell, Z. T. The space between notes: emerging roles for translationally silent ribosomes. Trends Biochem. Sci 47, 477–491 (2022).
Beckert, B. et al. Structure of a hibernating 100S ribosome reveals an inactive conformation of the ribosomal protein S1. Nat. Microbiol. 3, 1115–1121 (2018).
Beckert, B. et al. Structure of the Bacillus subtilis hibernating 100S ribosome reveals the basis for 70S dimerization. EMBO J. 36, 2061–2072 (2017).
Barandun, J., Hunziker, M., Vossbrinck, C. R. & Klinge, S. Evolutionary compaction and adaptation visualized by the structure of the dormant microsporidian ribosome. Nat. Microbiol. 4, 1798–1804 (2019).
Brown, A., Baird, M. R., Yip, M. C., Murray, J. & Shao, S. Structures of translationally inactive mammalian ribosomes. eLife 7, e40486 (2018).
Van Dyke, N., Baby, J. & Van Dyke, M. W. Stm1p, a ribosome-associated protein, is important for protein synthesis in Saccharomyces cerevisiae under nutritional stress conditions. J. Mol. Biol. 358, 1023–1031 (2006).
Smith, P. R. et al. Functionally distinct roles for eEF2K in the control of ribosome availability and p-body abundance. Nat. Commun. 12, 6789 (2021).
Shetty, S., Hofstetter, J., Battaglioni, S., Ritz, D. & Hall, M. N. TORC1 phosphorylates and inhibits the ribosome preservation factor Stm1 to activate dormant ribosomes. Preprint at https://doi.org/10.1101/2022.08.08.503151 (2022).
Wells, J. N. et al. Structure and function of yeast Lso2 and human CCDC124 bound to hibernating ribosomes. PLoS Biol. 18, e3000780 (2020).
Seefeldt, A. C. et al. Structure of the mammalian antimicrobial peptide Bac7(1–16) bound within the exit tunnel of a bacterial ribosome. Nucleic Acids Res. 44, 2429–2438 (2016).
Casteels, P., Ampe, C., Jacobs, F., Vaeck, M. & Tempst, P. Apidaecins: antibacterial peptides from honeybees. EMBO J. 8, 2387–2391 (1989).
Krizsan, A., Prahl, C., Goldbach, T., Knappe, D. & Hoffmann, R. Short proline-rich antimicrobial peptides inhibit either the bacterial 70S ribosome or the assembly of its large 50S subunit. ChemBioChem 16, 2304–2308 (2015).
Metafora, S., Felicetti, L. & Gambino, R. The mechanism of protein synthesis activation after fertilization of sea urchin eggs. Proc. Natl Acad. Sci. USA 68, 600–604 (1971).
Gambino, R., Metafora, S., Felicetti, L. & Raisman, J. Properties of the ribosomal salt wash from unfertilized and fertilized sea urchin eggs and its effect on natural mRNA translation. Biochim. Biophys. Acta 312, 377–391 (1973).
Hille, M. B. Inhibitor of protein synthesis isolated from ribosomes of unfertilised eggs and embryos of sea urchins. Nature 249, 556–558 (1974).
Chassé, H., Boulben, S., Costache, V., Cormier, P. & Morales, J. Analysis of translation using polysome profiling. Nucleic Acids Res. 45, e15 (2017).
Chew, G.-L. et al. Ribosome profiling reveals resemblance between long non-coding RNAs and 5′ leaders of coding RNAs. Development 140, 2828–2834 (2013).
Pauli, A. et al. Toddler: an embryonic signal that promotes cell movement via apelin receptors. Science 343, 1248636 (2014).
Gutierrez, E. et al. eIF5A promotes translation of polyproline motifs. Mol. Cell 51, 35–45 (2013).
Schuller, A. P., Wu, C. C.-C., Dever, T. E., Buskirk, A. R. & Green, R. eIF5A functions globally in translation elongation and termination. Mol. Cell 66, 194–205.e5 (2017).
Schmidt, C. et al. Structure of the hypusinylated eukaryotic translation factor eIF-5A bound to the ribosome. Nucleic Acids Res. 44, 1944–1951 (2016).
Rodnina, M. V., Savelsbergh, A., Katunin, V. I. & Wintermeyer, W. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature 385, 37–41 (1997).
Flis, J. et al. tRNA translocation by the eukaryotic 80S ribosome and the Impact of GTP hydrolysis. Cell Rep. 25, 2676–2688.e7 (2018).
Hayashi, H. et al. Tight interaction of eEF2 in the presence of Stm1 on ribosome. J. Biochem. 163, 177–185 (2018).
Anger, A. M. et al. Structures of the human and Drosophila 80S ribosome. Nature 497, 80–85 (2013).
Sun, L., Ryan, D. G., Zhou, M., Sun, T.-T. & Lavker, R. M. EEDA: a protein associated with an early stage of stratified epithelial differentiation. J. Cell. Physiol. 206, 103–111 (2006).
Ma, X. et al. Regulation of cell proliferation in the retinal pigment epithelium: differential regulation of the death-associated protein like-1 DAPL1 by alternative MITF splice forms. Pigment Cell Melanoma Res. 31, 411–422 (2018).
Ma, X. et al. DAPL1, a susceptibility locus for age-related macular degeneration, acts as a novel suppressor of cell proliferation in the retinal pigment epithelium. Hum. Mol. Genet. 26, 1612–1621 (2017).
Deiss, L. P., Feinstein, E., Berissi, H., Cohen, O. & Kimchi, A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the γ interferon-induced cell death. Genes Dev. 9, 15–30 (1995).
Koren, I., Reem, E. & Kimchi, A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Curr. Biol. 20, 1093–1098 (2010).
Saini, P., Eyler, D. E., Green, R. & Dever, T. E. Hypusine-containing protein eIF5A promotes translation elongation. Nature 459, 118–121 (2009).
Park, M. H., Nishimura, K., Zanelli, C. F. & Valentini, S. R. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38, 491–500 (2010).
Greber, B. J., Boehringer, D., Montellese, C. & Ban, N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat. Struct. Mol. Biol. 19, 1228–1233 (2012).
Klingauf-Nerurkar, P. et al. The GTPase Nog1 co-ordinates the assembly, maturation and quality control of distant ribosomal functional centers. eLife 9, e52474 (2020).
Zhong, E. D., Bepler, T., Berger, B. & Davis, J. H. CryoDRGN: reconstruction of heterogeneous cryo-EM structures using neural networks. Nat. Methods 18, 176–185 (2021).
Rossi, D. et al. Evidence for a negative cooperativity between eIF5A and eEF2 on binding to the ribosome. PLoS ONE 11, e0154205 (2016).
Kao, A. et al. Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol. Cell. Proteomics 10, M110.002212 (2011).
Balagopal, V. & Parker, R. Stm1 modulates translation after 80S formation in Saccharomyces cerevisiae. RNA 17, 835–842 (2011).
Blobel, G. & Potter, V. R. Studies on free and membrane-bound ribosomes in rat liver: I. Distribution as related to total cellular RNA. J. Mol. Biol. 26, 279–292 (1967).
Marygold, S. J. et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol, 8, R216 (2007).
Fortier, S., MacRae, T., Bilodeau, M., Sargeant, T. & Sauvageau, G. Haploinsufficiency screen highlights two distinct groups of ribosomal protein genes essential for embryonic stem cell fate. Proc. Natl Acad. Sci. USA 112, 2127–2132 (2015).
Amsterdam, A. et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2, E139 (2004).
Vecchi, G. et al. Proteome-wide observation of the phenomenon of life on the edge of solubility. Proc. Natl Acad. Sci. USA 117, 1015–1020 (2020).
Liu, Y. et al. Autophagy-dependent ribosomal RNA degradation is essential for maintaining nucleotide homeostasis during C. elegans development. eLife 7, e36588 (2018).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences https://doi.org/10.4324/9780203771587 (Routledge, 1988).
Juszkiewicz, S. et al. ZNF598 is a quality control sensor of collided ribosomes. Mol. Cell 72, 469–481.e7 (2018).
Li, W. et al. Structural basis for selective stalling of human ribosome nascent chain complexes by a drug-like molecule. Nat. Struct. Mol. Biol. 26, 501–509 (2019).
Chandrasekaran, V. et al. Mechanism of ribosome stalling during translation of a poly(A) tail. Nat. Struct. Mol. Biol. 26, 1132–1140 (2019).
Gagnon, J. A. et al. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9, e98186 (2014).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Nair, S., Lindeman, R. E. & Pelegri, F. In vitro oocyte culture-based manipulation of zebrafish maternal genes. Dev. Dyn. 242, 44–52 (2013).
Sive, H. L., Grainger, R. M. & Harland, R. M. Early Development of Xenopus laevis (Cold Spring Harbor Laboratory Press, 2000).
Khatter, H. et al. Purification, characterization and crystallization of the human 80S ribosome. Nucleic Acids Res. 42, e49 (2014).
Dorfer, V. et al. MS Amanda, a universal identification algorithm optimized for high accuracy tandem mass spectra. J. Proteome Res. 13, 3679–3684 (2014).
Käll, L., Canterbury, J. D., Weston, J., Noble, W. S. & MacCoss, M. J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 (2007).
Taus, T. et al. Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10, 5354–5362 (2011).
Doblmann, J. et al. apQuant: accurate label-free quantification by quality filtering. J. Proteome Res. 18, 535–541 (2019).
Smyth, G. K. in Bioinformatics and Computational Biology Solutions Using R and Bioconductor (eds. Gentleman, R. et al.) 397–420 (Springer, 2005).
Pirklbauer, G. J. et al. MS Annika: a new cross-linking search engine. J. Proteome Res. 20, 2560–2569 (2021).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Sharma, A., Mariappan, M., Appathurai, S. & Hegde, R. S. In vitro dissection of protein translocation into the mammalian endoplasmic reticulum. Methods Mol. Biol. 619, 339–363 (2010).
Feng, Q. & Shao, S. In vitro reconstitution of translational arrest pathways. Methods 137, 20–36 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Yang, H. et al. Automated and accurate deposition of structures solved by X-ray diffraction to the Protein Data Bank. Acta Crystallogr. D 60, 1833–1839 (2004).
Cabrera-Quio, L. E., Schleiffer, A., Mechtler, K. & Pauli, A. Zebrafish Ski7 tunes RNA levels during the oocyte-to-embryo transition. PLoS Genet. 17, e1009390 (2021).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Session, A. M. et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336–343 (2016).
Fujihara, Y. et al. The conserved fertility factor SPACA4/Bouncer has divergent modes of action in vertebrate fertilization. Proc. Natl Acad. Sci. USA 118, e2108777118 (2021).
Gagnon, M. G. et al. Structures of proline-rich peptides bound to the ribosome reveal a common mechanism of protein synthesis inhibition. Nucleic Acids Res. 44, 2439–2450 (2016).
Florin, T. et al. An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat. Struct. Mol. Biol. 24, 752–757 (2017).
Kargas, V. et al. Mechanism of completion of peptidyltransferase centre assembly in eukaryotes. eLife 8, e44904 (2019).
Wu, S. et al. Diverse roles of assembly factors revealed by structures of late nuclear pre-60S ribosomes. Nature 534, 133–137 (2016).
Polikanov, Y. S., Steitz, T. A. & Innis, C. A. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat. Struct. Mol. Biol. 21, 787–793 (2014).
Acknowledgements
We thank L. E. Cabrera-Quio and M. Novatchkova for ribosome profiling data analysis; K. R. Gert for providing non-activated zebrafish eggs; the Mass Spectrometry Facility at the Vienna BioCenter Core Facilities (VBCF), in particular S. Opravil for the processing of our samples, and R. Imre and G. Dürnberger for their help with data analysis; the VBCF Protein Technologies facility, in particular D. Drechsel and A. Lehner, for the expression and purification of recombinant Dap1b and Dap proteins; M. Madalinski for synthesizing the Bac7, Dap(71–105) and Dap1b(76–109) peptides; A. Schleiffer for his help in the phylogenetic analysis of Dap and Dap1b/Dapl1; the VBCF Electron Microscopy Facility for the support and maintenance of the facilities; H. Stark (MPI BPC) for providing additional Krios measurement time; the IMP animal facility personnel, in particular F. Ecker, J. König and D. Sunjic for their excellent care of fish and Xenopus; M. Binner, J. Kiraly, T. Mylenko and A. Bandura for their help with genotyping; L. Leiendecker and the entire Obenauf laboratory for discussions and sharing reagents; E. Tanaka and T. Clausen for funding T.-Y.L. and A.M., respectively; the entire Pauli group for valuable discussions on the project; the VBC RNA Salon and the RNA-Deco-SFB community for providing useful feedback and suggestions; and A. Andersen, C. Plaschka, A. Stark, J. Brennecke, A. Carter, F. Peske, U. Hohmann, E. Calo, A. Shah and A. Blaha for critical reading and valuable feedback on the manuscript. This work was supported by the Institute of Molecular Pathology (IMP), which receives institutional funding from Boehringer Ingelheim and the Austrian Research Promotion Agency (Headquarter grant FFG-852936), and funding from the FWF START program (Y 1031-B28 to A.P.), the Human Frontier Science Program (HFSP) Career Development Award (CDA00066/2015 to A.P.), the SFB RNA-Deco (project number F 80 to A.P.) and EMBO-YIP funds (to A.P.). L.L.-O. was supported by an SNF Early Postdoc Mobility fellowship (P2GEP3_191204), an EMBO long-term fellowship (ALTF 1165-2019) and an MSCA-IF-EF-SE (890218). A.C. was supported by an MSCA-IF-EF-SE and a VIP2 postdoctoral fellowship. We acknowledge the cryo-EM facility CEITEC MU of CIISB, Instruct-CZ Centre, supported by MEYS CR (LM2018127), and Diamond Light Source for granting us access and support at the cryo-EM facilities at the UK’s National Electron Bio-imaging Centre (eBIC) under proposal EM BI25222, funded by the Wellcome Trust, MRC and BBSRC. For the purpose of Open Access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
F.L., L.L.-O., D.H. and A.P. conceived the study. F.L. performed most experiments with the help of C.P., further supported by J.R. and A.C. L.L.-O. obtained the final cryo-EM maps and modelled all ribosomes with contributions of F.L., A.M. and D.H. and help from S.K. I.G. prepared and screened grids, collected EM data, and together with F.L. and D.H., contributed to the initial cryo-EM data processing with help from K.B. M.M. and E.R. performed and analysed the crosslinking sample preparation, and K.M. supervised mass spectrometry experiments and analysed the crosslinking samples. T.-Y.L. provided Xenopus eggs and 24 hpf embryos. D.H. performed the cryoDRGN analysis and F.L. and L.L-O. compared the resulting maps. A.P. and D.H. supervised the project. L.L.-O., F.L. and A.P. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Antonio Giraldez, Daniel Wilson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Mass-spectrometry and cryo-EM analysis of Xenopus egg ribosomes.
a, Fold change of ribosome-associated factors and core ribosomal proteins after comparing mass spectrometry data of purified ribosomes from unfertilized Xenopus eggs and 24 hpf larvae (stage 14) (n = 1 experiment). b, Processing pipeline of the Xenopus egg ribosome. All steps were done in Cryosparc v3.2.0. Maps are shown in grey, masks in blue. c-d, Maps showing the local resolution of Map1 and Map2 (c), and of the four factors associated with Xenopus egg ribosomes (d). Note that the resolution of the large subunit in Map2 (in c) is 0 (shown in blue) since this region was outside the mask used for obtaining this map (see b). e, Orientation distribution plot for all particles contributing to Map1. f, Gold-Standard Fourier Shell Correlation (GSFSC) of Map1.
Extended Data Fig. 2 Processing pipeline of the 1 hpf zebrafish ribosome.
All steps were done in Cryosparc v3.2.0. Maps are shown in grey, masks in blue. The orientation distribution plot for all particles contributing to Map1 and the Gold-Standard Fourier Shell Correlation (GSFSC) of the respective map is shown on the bottom. Local resolution maps were calculated for Map1, Map2, Map3, and for the four ribosome-associated factors. Note that in the box with local resolution maps, the resolution of the large subunit in Map2 and Map3, and of the small subunit’s head in Map3 is 0 (shown in blue) since these regions were outside the masks used for generating these maps.
Extended Data Fig. 3 Characterization of the dormant ribosome state in zebrafish 1 hpf and Xenopus egg ribosomes.
a-d, Densities of the two modules, namely Habp4-eEF2b/eEF2 (a-b), and Dap1b/Dapl1-eIF5a (c-d), that are characteristic for dormant ribosomes in zebrafish 1 hpf embryos and Xenopus eggs. e, Overview of the ribosome structure isolated from 6 hpf zebrafish embryos lacking the specific egg ribosome-associated factors. f, Latent space representations of ribosomal particles from Xenopus eggs (left), 1 hpf zebrafish embryos (middle) and 6 hpf zebrafish embryos (right) as UMAP embeddings after training a cryoDRGN latent variable model. Classes are depicted in Roman numbers, map volumes are indicated with Arabic numbers. Total particle numbers are shown on the top left of each graph. g, Densities of ribosome-associated factors from Xenopus eggs. An overview of the map is shown on the top left. h, Densities of ribosome-associated factors in a map reconstructed from class I particles from Xenopus eggs obtained with cryoDRGN. An overview of the map is shown on the top-left.
Extended Data Fig. 4 Processing pipeline of the 6 hpf zebrafish ribosome.
All steps were done in Cryosparc v3.2.0. Maps are shown in grey, masks in blue. The orientation distribution plot for all particles contributing to Map1 and the Gold-Standard Fourier Shell Correlation (GSFSC) of the respective map is shown on the bottom-right. Local resolution maps were calculated for Map1, Map2 and Map3. Note that in the box with local resolution maps, the resolution of the large subunit in Map2 and Map3, and of the small subunit’s body in Map3 is 0 (shown in blue) since these regions were outside the masks used for generating these maps.
Extended Data Fig. 5 Sequence conservation of the Dap/Dap1b/Dapl1 protein family and RNA expression of ribosome-associated factors.
a, Protein sequence alignment of the Dap/Dap1b/Dapl1 protein family illustrates conserved motifs. Vertebrates have two paralogs, namely Dap1b/Dapl1 and Dap. Invertebrates only encode one homolog (Dap1) that clusters in between Dap1b/Dapl1 and Dap proteins. b, Zebrafish mRNA expression levels (PolyA+ RNA-seq31,82) of eif5a/eif5a2 (purple), eef2b (orange), dap1b/dap (green) and habp4 (blue) during oogenesis and embryogenesis. c, Xenopus mRNA expression levels of all paralogs of eif5a, eef2, dap, dapl1 and habp4 derived from riboMinus-seq data84. d, mRNA expression levels of zebrafish eif5a/eif5a2, eef2b, dap1b/dap and habp4 in adult tissues85. TPM, transcripts per million.
Extended Data Fig. 6 Structural comparison of Dap1b and other factors that insert into the polypeptide exit tunnel (PET).
a, Structures of ribosomes with proteins and peptides inserted into the PET. From left to right: zebrafish Dap1b inserted into the rabbit ribosome, Bac7 (5HAU86), Api137 (5O2R87), Rei1 (6RZZ88), Nog1 (3JCT89) and MDF2 (6RM317). Models were clipped to have a better view of the PET. Boxed areas (dashed boxes) are shown at higher magnification in b. b, Detail of the peptidyl-transferase center (PTC) of the ribosomes shown in a. The dashed line indicates the position of the PTC. All previously known factors use different mechanisms than Dap1b to achieve their functions: Bac7 interacts with the ribosomal A-loop via its N terminus (see below) to block translation initiation, Api137 interacts with the release factors RF1 or RF2 to block termination of bacterial ribosomes, Rei1 and Nog1 insert into the PET of 60S subunits during ribosome biogenesis and their C termini do not extend beyond the PTC, and MDF2’s C terminus interferes with P-tRNA and eIF5A binding to establish dormancy in the microsporidian ribosome. c, Superimposition of Dap1b (left) and Bac7 (right) with eukaryotic (5GAK34) and prokaryotic (1VY490) A- and P-tRNAs, respectively. Red asterisks denote clashes of Dap1b and Bac7 with the A-tRNA. d, Dap1b’s C terminus does not interact with the A-loop (right), in contrast to Bac7 (left). Dashed lines mark distances between Arg1 of Bac7 and Phe109 of Dap1b with a conserved uracil of the A-loop. e, Scheme of the interactions of Dap1b’s C terminus within the ribosome. Dap1b interacts with helix 74 (H74) and H90 of the 28S rRNA. f, Scheme of the interactions of Bac7’s N terminus within the ribosome. Bac7 interacts with H89 and the A-loop (H92).
Extended Data Fig. 7 Crosslinking and mass spectrometry (MS) analysis of ribosomes from 1 hpf zebrafish embryos and Xenopus eggs.
a, Cα-Cα distance distribution of the DSSO-induced crosslinks identified in Xenopus egg and zebrafish 1 hpf ribosomes. b-c, Proteins crosslinked to zebrafish Habp4 (b) or Dap (c) are shown on the 1 hpf zebrafish ribosome as surface representations, with crosslinked residues depicted in darker color. d, Crosslinking mapping of Habp4 (shown as a scheme; modeled regions highlighted with a black line) to proteins of the zebrafish embryo (top) and Xenopus egg (bottom) ribosome. Crosslinked proteins are shown as surface representations, with crosslinked residues depicted in dark blue. A cartoon (right) shows a model of Habp4 on the ribosome. e, Crosslinking mapping of zebrafish Dap (top) and Xenopus Dapl1 (bottom; for details, see d).
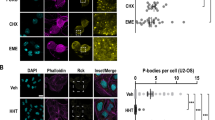
Extended Data Fig. 8 Characterization of single (habp4−/−), double, and triple (dap−/− dap1b−/− habp4−/−) zebrafish mutants.
a-b, Early embryo development of habp4−/− (a) and dap−/ − dap1b−/− mutants (b) compared to wildtype (WT). c, Mendelian ratio analysis of fin-clips from adult fish obtained from heterozygous habp4+/− parents (25% expected to be habp4−/−; n = 4 for both genotypes). d, Size of WT and habp4−/− embryos at 6 h post-fertilization (hpf) (WT-1: n = 31; WT-2: n = 22; WT-3: n = 35; habp4−/−−1: n = 36; habp4−/−−2: n = 24; habp4−/−−3: n = 33). e, Total RNA of 1–3 hpf embryos (WT: n = 42; dap−/− dap1b−/−: n = 41; WT and triple KO: n = 20 for both genotypes). f, Number of eggs laid by single, double, and triple KO females compared to matching WT. g, Representative images (left) and quantification (right) of poor quality eggs. h, Percentage of embryos from single, double, and triple KO mutant pairs displaying normal embryo development until 6 hpf compared to matching WT pairs. i-j, Relative number of embryos and larvae (in relation to the embryos that developed normally up to 6 hpf; see h) that showed abnormalities at 1 (i) and 4 (j) days post-fertilization (dpf). Example images are shown on the left. Data in c and e-j are represented as scatter dot plots with means ± standard deviation (SD). Data in d are represented as a violin plot with median and quartiles. In f-h, dotted vertical lines indicate separate experiments. Significance was determined using Kruskal-Wallis and Dunn’s two-sided test (d, f-j; for more than 2 sample group comparisons) or Mann-Whitney test (c, and in e-h for pairwise comparisons between habp4−/− or dap−/− dap1b−/− versus WT). For c and f, n are independent crosses; for d, n are individual embryos; for e, n are biologically independent samples. #, number of crosses.
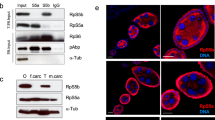
Extended Data Fig. 9 Recombinant Dap1b binds to the polypeptide exit tunnel of rabbit ribosomes.
a-b, Western blot of the total in vitro translation reaction shown in Fig. 4c (a) and Fig. 4e (b). Uncropped images of membranes are provided in Supplementary Fig. 2a, b. c, Translation activity assays (Fig. 4a top) of renilla luciferase mRNA upon addition of increasing concentrations of C-terminal Dap and Dap1b peptides. BSA and N-terminal Bac7 are used as negative and positive controls, respectively (n = 4 biologically independent samples). Dots represent means and error bars are standard deviation (SD). d, Ribosome binding assays (Fig. 4a bottom) of in vitro translated FLAG-tagged Dap-Dap1b chimeras compared to full-length (WT) Dap and Dap1b. A representative Western blot from a single experiment is shown on the left; quantification of three independent experiments is shown on the right. Data are represented as scatter dot plots with means ± standard deviation (SD). Significance was assessed with Kruskal-Wallis followed by Dunn’s two-sided test. Uncropped images of membranes are provided in Supplementary Fig. 2d. e, Mass spectrometry data of ribosomes isolated from habp4−/− and WT (left), and from dap−/− dap1b−/− and WT (right) embryos at 1 hpf, represented as volcano plots (n = 3 independent experiments). Permutation-based false discovery rates (FDRs) are displayed as dotted (FDR < 0.01) and dashed (FDR < 0.05) lines.
Extended Data Fig. 10 Processing pipeline of the rabbit ribosome with recombinant zebrafish Dap1b.
a, Processing pipeline for obtaining an 80S density map of the rabbit ribosome with zebrafish Dap1b. b, Orientation distribution plot (top) and Gold-Standard Fourier Shell Correlation (GSFSC; bottom) of Map1. c, Local resolution maps calculated for Map2 and Map3. Densities and local resolutions of eIF5A, Dap1b, eEF2 and SERBP1 are shown on the bottom. Note that the resolution of the small subunit in Map2 is 0 (shown in blue) since this region was outside the mask used for generating this map. d, Latent space representation of particles from rabbit ribosomes with zebrafish Dap1b as a UMAP embedding after training a cryoDRGN latent variable model. Classes are depicted in Roman numbers, map volumes are indicated with Arabic numbers. Total particle number is shown on the top left of the graph.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1 and 2, Supplementary Tables 2 and 5 and full descriptions for Supplementary Tables 1, 3 and 4 (supplied separately).
Supplementary Data
Source data for Supplementary Fig. 1.
Supplementary Table 1
Proteins differentially associated with ribosomes. Proteins significantly enriched or depleted in ribosomes isolated from 1 hpf zebrafish embryos versus zebrafish eggs, 3 hpf and 6 hpf embryos (permutation-based FDR <0.05)
Supplementary Table 3
CryoDRGN analysis of ribosome particles. Classification was done based on the presence of eEF2, eIF5a and tRNA factors.
Supplementary Table 4
Dap, Dap1b/Dapl1 and Habp4 crosslinks. Crosslinks identified in ribosome samples from 1 hpf zebrafish embryos and Xenopus eggs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leesch, F., Lorenzo-Orts, L., Pribitzer, C. et al. A molecular network of conserved factors keeps ribosomes dormant in the egg. Nature 613, 712–720 (2023). https://doi.org/10.1038/s41586-022-05623-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05623-y
This article is cited by
-
eIF4E1b is a non-canonical eIF4E protecting maternal dormant mRNAs
EMBO Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.