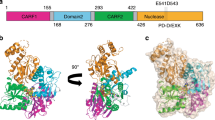
Abstract
CRISPR defence systems such as the well-known DNA-targeting Cas9 and the RNA-targeting type III systems are widespread in prokaryotes1,2. The latter orchestrates a complex antiviral response that is initiated through the synthesis of cyclic oligoadenylates after recognition of foreign RNA3,4,5. Among the large set of proteins that are linked to type III systems and predicted to bind cyclic oligoadenylates6,7, a CRISPR-associated Lon protease (CalpL) stood out to us. CalpL contains a sensor domain of the SAVED family7 fused to a Lon protease effector domain. However, the mode of action of this effector is unknown. Here we report the structure and function of CalpL and show that this soluble protein forms a stable tripartite complex with two other proteins, CalpT and CalpS, that are encoded on the same operon. After activation by cyclic tetra-adenylate (cA4), CalpL oligomerizes and specifically cleaves the MazF homologue CalpT, which releases the extracytoplasmic function σ factor CalpS from the complex. Our data provide a direct connection between CRISPR-based detection of foreign nucleic acids and transcriptional regulation. Furthermore, the presence of a SAVED domain that binds cyclic tetra-adenylate in a CRISPR effector reveals a link to the cyclic-oligonucleotide-based antiphage signalling system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The crystal structures have been deposited into the Protein Data Bank database with the accession codes 7QDA, 8B0R and 8B0U. The SAXS data and models have been deposited into the Small Angle Scattering Biological Data Bank with the accession codes SASDQM4, SASDQN4, SASDQP4 and SASDQQ4. The following Protein Data Bank entries were used in this study: 2H27, 3K1J, 4ME7, 4IZJ, 4LUP, 5ZX2, 5CR2, 6VM6, 6SCE and 7RWK. Source data are provided with this paper.
Code availability
No custom code was used in this work.
References
Makarova, K. S., Wolf, Y. I. & Koonin, E. V. Classification and nomenclature of CRISPR–Cas systems: where from here. CRISPR J. 1, 325–336 (2018).
Zhu, Y., Klompe, S. E., Vlot, M., van der Oost, J. & Staals, R. H. J. Shooting the messenger: RNA-targetting CRISPR–Cas systems. Biosci. Rep. 38, BSR20170788 (2018).
Kazlauskiene, M., Kostiuk, G., Venclovas, Č., Tamulaitis, G. & Siksnys, V. A cyclic oligonucleotide signaling pathway in type III CRISPR–Cas systems. Science 357, 605–609 (2017).
Niewoehner, O. et al. Type III CRISPR–Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017).
Rouillon, C., Athukoralage, J. S., Graham, S., Grüschow, S. & White, M. F. Control of cyclic oligoadenylate synthesis in a type III CRISPR system. eLife 7, e36734 (2018).
Shmakov, S. A., Makarova, K. S., Wolf, Y. I., Severinov, K. V. & Koonin, E. V. Systematic prediction of genes functionally linked to CRISPR–Cas systems by gene neighborhood analysis. Proc. Natl Acad. Sci. USA 115, E5307–E5316 (2018).
Shah, S. A. et al. Comprehensive search for accessory proteins encoded with archaeal and bacterial type III CRISPR–cas gene cassettes reveals 39 new cas gene families. RNA Biol. 16, 530–542 (2019).
Gasiunas, G., Sinkunas, T. & Siksnys, V. Molecular mechanisms of CRISPR-mediated microbial immunity. Cell. Mol. Life Sci. 71, 449–465 (2014).
Sasnauskas, G. & Siksnys, V. CRISPR adaptation from a structural perspective. Curr. Opin. Struct. Biol. 65, 17–25 (2020).
Jiang, F. & Doudna, J. A. CRISPR–Cas9 structures and mechanisms. Annu. Rev. Biophys. 46, 505–529 (2017).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Athukoralage, J. S. & White, M. F. Cyclic oligoadenylate signalling and regulation by ring nucleases during type III CRISPR defence. RNA 27, 855–867 (2021).
Jia, N., Jones, R., Sukenick, G. & Patel, D. J. Second messenger cA4 formation within the composite Csm1 Palm pocket of type III-A CRISPR–Cas Csm complex and its release path. Mol. Cell 75, 933–943.e6 (2019).
Makarova, K. S., Anantharaman, V., Grishin, N. V., Koonin, E. V. & Aravind, L. CARF and WYL domains: ligand-binding regulators of prokaryotic defense systems. Front. Genet. 5, 102 (2014).
Lau, R. K. et al. Structure and mechanism of a cyclic trinucleotide-activated bacterial endonuclease mediating bacteriophage immunity. Mol. Cell 77, 723–733.e6 (2020).
Lintner, N. G. et al. The structure of the CRISPR-associated protein Csa3 provides insight into the regulation of the CRISPR/Cas system. J. Mol. Biol. 405, 939–955 (2011).
McMahon, S. A. et al. Structure and mechanism of a type III CRISPR defence DNA nuclease activated by cyclic oligoadenylate. Nat. Commun. 11, 500 (2020).
Rostøl, J. T. et al. The Card1 nuclease provides defence during type III CRISPR immunity. Nature 590, 624–629 (2021).
Garcia-Doval, C. et al. Activation and self-inactivation mechanisms of the cyclic oligoadenylate-dependent CRISPR ribonuclease Csm6. Nat. Commun. 11, 1596 (2020).
Lawrence, C. M., Charbonneau, A. & Gauvin, C. Cyclic tetra‐adenylate (cA4) activates CRISPR associated transcription factor Csa3, providing feedback activation of protospacer acquisition and crRNA expression. FASEB J. 34, 1–1 (2020).
Meeske, A. J., Nakandakari-Higa, S. & Marraffini, L. A. Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 570, 241–245 (2019).
Burroughs, A. M., Zhang, D., Schäffer, D. E., Iyer, L. M. & Aravind, L. Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling. Nucleic Acids Res. 43, 10633–10654 (2015).
Lowey, B. et al. CBASS immunity uses CARF-related effectors to sense 3′-5′- and 2′-5′-linked cyclic oligonucleotide signals and protect bacteria from phage infection. Cell 182, 38–49.e17 (2020).
Makarova, K. S. et al. Evolutionary and functional classification of the CARF domain superfamily, key sensors in prokaryotic antivirus defense. Nucleic Acids Res. 48, 8828–8847 (2020).
Zwart, P. H. et al. Automated structure solution with the PHENIX suite. Methods Mol. Biol. 426, 419–435 (2008).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Chung, I. Y. & Paetzel, M. Crystal structures of yellowtail ascites virus VP4 protease: trapping an internal cleavage site trans acyl–enzyme complex in a native Ser/Lys dyad active site. J. Biol. Chem. 288, 13068–13081 (2013).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. L. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Fatma, S., Chakravarti, A., Zeng, X. & Huang, R. H. Molecular mechanisms of the CdnG–Cap5 antiphage defense system employing 3′, 2′-cGAMP as the second messenger. Nat. Commun. 12, 6381 (2021).
Jiang, K. et al. Structural basis of formation of the microtubule minus-end-regulating CAMSAP–katanin complex. Structure 26, 375–382.e4 (2018).
Saha, C. K., Sanches Pires, R., Brolin, H., Delannoy, M. & Atkinson, G. C. FlaGs and webFlaGs: discovering novel biology through the analysis of gene neighbourhood conservation. Bioinformatics 37, 1312–1314 (2021).
Zimmermann, L. et al. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010).
Simanshu, D. K., Yamaguchi, Y., Park, J.-H., Inouye, M. & Patel, D. J. Structural basis of mRNA recognition and cleavage by toxin MazF and its regulation by antitoxin MazE in Bacillus subtilis. Mol. Cell 52, 447–458 (2013).
Hogrel, G. et al. Cyclic nucleotide-induced helical structure activates a TIR immune effector. Nature 608, 808–812 (2022).
Paget, M. S. Bacterial sigma factors and anti-sigma factors: structure, function and distribution. Biomolecules 5, 1245–1265 (2015).
Sineva, E., Savkina, M. & Ades, S. E. Themes and variations in gene regulation by extracytoplasmic function (ECF) sigma factors. Curr. Opin. Microbiol. 36, 128–137 (2017).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Schuster, C. F. & Bertram, R. Toxin–antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiol. Lett. 340, 73–85 (2013).
Walsh, P. N. & Ahmad, S. S. Proteases in blood clotting. Essays Biochem. 38, 95–112 (2002).
Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 (2000).
Fei, X., Bell, T. A., Barkow, S. R., Baker, T. A. & Sauer, R. T. Structural basis of ClpXP recognition and unfolding of ssrA-tagged substrates. eLife 9, e61496 (2020).
Hu, C. et al. Craspase is a CRISPR RNA-guided, RNA-activated protease. Science 377, 1278–1285 (2022).
van Beljouw, S. P. B. et al. The gRAMP CRISPR–Cas effector is an RNA endonuclease complexed with a caspase-like peptidase. Science 373, 1349–1353 (2021).
Kato, K. et al. RNA-triggered protein cleavage and cell growth arrest by the type III-E CRISPR nuclease-protease. Science 378, 882–889 (2022).
Strecker, J. et al. RNA-activated protein cleavage with a CRISPR-associated endopeptidase. Science 378, 874–881 (2022).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Liu, H. & Naismith, J. H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 8, 91 (2008).
Rouillon, C., Athukoralage, J. S., Graham, S., Grüschow, S. & White, M. F. Investigation of the cyclic oligoadenylate signaling pathway of type III CRISPR systems. Methods Enzymol. 616, 191–218 (2019).
Cianci, M. et al. P13, the EMBL macromolecular crystallography beamline at the low-emittance PETRA III ring for high-and low-energy phasing with variable beam focusing. J. Synchrotron Radiat. 24, 323–332 (2017).
Kabsch, W. Automatic-indexing of rotation diffraction patterns. J. Appl. Crystallogr. 21, 67–72 (1988).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Williams, C. J. et al. MolProbity: more and better reference data for improved all‐atom structure validation. Protein Sci. 27, 293–315 (2018).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Blanchet, C. E. et al. Versatile sample environments and automation for biological solution X-ray scattering experiments at the P12 beamline (PETRA III, DESY). J. Appl. Crystallogr. 48, 431–443 (2015).
Graewert, M. A. et al. Adding size exclusion chromatography (SEC) and light scattering (LS) devices to obtain high-quality small angle X-ray scattering (SAXS) data. Crystals 10, 975 (2020).
Franke, D., Kikhney, A. G. & Svergun, D. I. Automated acquisition and analysis of small angle X-ray scattering data. Nucl. Instrum. Methods Phys. Res. A 689, 52–59 (2012).
Konarev, P. V., Volkov, V. V., Sokolova, A. V., Koch, M. H. J. & Svergun, D. I. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 36, 1277–1282 (2003).
Manalastas-Cantos, K. et al. ATSAS 3.0: expanded functionality and new tools for small-angle scattering data analysis. J. Appl. Crystallogr. 54, 343–355 (2021).
Svergun, D., Barberato, C. & Koch, M. H. J. CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 28, 768–773 (1995).
Franke, D. & Svergun, D. I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 42, 342–346 (2009).
Svergun, D. I. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 76, 2879–2886 (1999).
Volkov, V. V. & Dmitri, I. S. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 36, 860–864 (2003).
Kozin, M. B. & Svergun, D. I. Automated matching of high- and low-resolution structural models. J. Appl. Crystallogr. 34, 33–41 (2001).
Petoukhov, M. V. et al. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Crystallogr. 45, 342–350 (2012).
Milov, A., Salikohov, K. & Shirov, M. Application of Endor in electron-spin echo for paramagnetic center space distribution in solids. Fizika Tverdogo Tela 23, 975–982 (1981).
Pannier, M., Veit, S., Godt, A., Jeschke, G. & Spiess, H. W. Dead-time free measurement of dipole–dipole interactions between electron spins. J. Magn. Reson. 142, 331–340 (2000).
Larsen, R. G. & Singel, D. J. Double electron–electron resonance spin–echo modulation: spectroscopic measurement of electron spin pair separations in orientationally disordered solids. J. Chem. Phys. 98, 5134–5146 (1993).
Worswick, S. G., Spencer, J. A., Jeschke, G. & Kuprov, I. Deep neural network processing of DEER data. Sci. Adv. 4, eaat5218 (2018).
Fábregas Ibáñez, L., Jeschke, G. & Stoll, S. DeerLab: a comprehensive software package for analyzing dipolar electron paramagnetic resonance spectroscopy data. Magn. Reson. 1, 209–224 (2020).
Jeschke, G., Chechik, V., Ionita, P. & Godt, A. DeerAnalysis2006—a comprehensive software package for analyzing pulsed ELDOR data. Appl. Magn. Reson. 30, 473–498 (2006).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Cha, S. S. et al. Crystal structure of Lon protease: molecular architecture of gated entry to a sequestered degradation chamber. EMBO J. 29, 3520–3530 (2010).
Chung, I. Y. W. & Paetzel, M. Crystal structure of a viral protease intramolecular acyl-enzyme complex. J. Biol. Chem. 286, 12475–12482 (2011).
Léa, M. C. et al. Bacterial RadA is a DnaB-type helicase interacting with RecA to promote bidirectional D-loop extension. Nat. Commun. 8, 15638 (2017).
Zorzini, V. et al. Substrate recognition and activity regulation of the Escherichia coli mRNA endonuclease MazF. J. Biol. Chem. 291, 10950–10960 (2016).
Hagelueken, G., Ward, R., Naismith, J. H. & Schiemann, O. MtsslWizard: in silico spin-labeling and generation of distance distributions in PyMOL. Appl. Magn. Reson. 42, 377–391 (2012).
Campagne, S., Marsh, M. E., Capitani, G., Vorholt, J. A. & Allain, F. H. T. Structural basis for −10 promoter element melting by environmentally induced sigma factors. Nat. Struct. Mol. Biol. 21, 269–276 (2014).
Lane, W. J. & Darst, S. A. The structural basis for promoter −35 element recognition by the group IV σ factors. PLoS Biol. 4, e269 (2006).
Li, L., Fang, C., Zhuang, N., Wang, T. & Zhang, Y. Structural basis for transcription initiation by bacterial ECF σ factors. Nat. Commun. 10, 1153 (2019).
Acknowledgements
The synchrotron MX data were collected at beamline P13, operated by staff at EMBL Hamburg at the PETRA III storage ring (DESY, Hamburg, Germany). We thank G. Bourenkov and I. Bento for the assistance in using the beamline; V. Siksnys for helpful discussions; S. Shirran and S. Synowsky for the MS analysis; N. Brenner for technical assistance; and M. Drag and J. Grzymska for discussions and an initial peptide screen. M.G. and J.L.S.-B. are funded by the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy–EXC2151–390873048. M.F.W. acknowledges a European Research Council Advanced Grant (grant number 101018608) and the China Scholarship Council (reference 202008420207 to H.C.). B.E.B. acknowledges EPR equipment funding by BBSRC(BB/R013780/1 and BB/T017740/1). G.H. is grateful for funding by the Deutsche Forschungsgemeinschaft (grant number HA6805/6-1).
Author information
Authors and Affiliations
Contributions
C.R. and G.H. conceived and supervised the study and performed initial protein expression and crystallization experiments using CalpL. R.S. and W.B. cloned the initial CalpL construct. N.S., C.R., M.F.W. and G.H. designed experiments. N.S. optimized the purification of CalpL and CalpT, crystallized CalpL, CalpL–cA4 and CalpL–CalpT10 and established and performed the cleavage assays, SEC–MALS and DLS experiments. N.S. and M.F.P. cloned all the mutant constructs. G.H. and N.S. solved and refined the CalpL, CalpL–cA4 and CalpL–CalpT10 structures. J.M. and M.G. designed and performed the SPR experiments. H.C. and M.F.W. planned and performed the ribonuclease assays. H.C., N.S. and M.F.W. cloned and purified CalpS and performed the binding and co-expression experiments involving CalpS. S.D.V. performed the SAXS experiments. S.D.V. and D.S. performed SAXS data analysis and interpretation and wrote the corresponding sections. B.E.B. and K.A. performed the pulsed EPR experiments, analysed the data, prepared figures and wrote the corresponding sections. K.B. and J.L.S.-B. designed and performed the RNase sequencing assays. C.R., M.F.W., H.C., N.S. and G.H. analysed the data and wrote the paper. All authors discussed the results and commented on the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Simon Jackson, David Taylor and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Purification and Structure of CalpL.
a, Gelfiltration chromatography (Superdex 200 16/60) of CalpL. Inset: SDS-PAGE analysis of the fractions indicated by the blue bar in the chromatogram. The experiment was performed multiple times (n > 3 biological replicates). b, TM-prediction by the TMHMM 2.0 server28 vs experimental structure. c, Representative electron density of the SeMet CalpL crystal structure. The structural model is drawn in ball-and-stick representation. Selected residues are labeled. The black mesh is a 2mFo-DFc electron density map contoured at 1.0 σ. d, Topology diagram of CalpL. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 CalpL in comparison to structurally related proteins.
a, CalpL is drawn as a cartoon model color-coded as in Fig. 1. The Lon protease from T. onnorineus (PDB-ID: 3K1J, DALI Z-score: 12.8)76 is shown as a white cartoon model. b, Table listing proteins with similar domain structures17,23,27,29,30,76,77,78. c, Surface electrostatics of the Lon protease active site region. The catalytic dyad is marked. The grey line marks the likely substrate binding site. d, Superposition of CalpL active site with the acyl-enzyme intermediate of yellowfin asciitis virus protease. CalpL is in sticks representation and color-coded as in Fig. 1. Chain D of structure 4IZJ27 (residues 630–640) was superimposed on the corresponding residues of CalpL (150-160) leading to an r. m. s. d. of 0.314 Å. Of 4IZJ, only the acyl-enzyme intermediate is shown in sticks mode. Selected residues and the positions of the P1-P3 sites are indicated. e, Superposition of CalpL (color scheme as in Fig. 1) with the Cap4 protein (white, PDB-ID: 6VM6)23. f, Superposition of the CalpL SAVED domain (color scheme as in Fig. 1) with the Can1 protein (white, PDB-ID: 6SCE)17. g, Superposition of the CalpL SAVED domain (color scheme as in Fig. 1) with the CARF domains of the Cap5 protein (white, PDB-ID: 7RWK)29.
Extended Data Fig. 3 The CalpL/cA4 complex.
a, Close-up of cA4 (green) bound to the SAVED domain of CalpL. The blue mesh is a 2mFo-DFc electron density map contoured at 1.0 σ. b, Superposition of CalpL apo (white) onto the cA4 complex structure (color coded as in Fig. 1). c, Structural alignment of the SAVED domains of CALP/cA4 and Cap4/cA3 (white).
Extended Data Fig. 4 CalpT is a MazF homolog and the target of the CalpL protease.
a, b, A superposition of the predicted CalpT structure (compare Fig. 2B) with one monomer of the MazF/ssRNA complex (purple/orange) (PDB-IDs: 5CR2)79. The AlphaFold233 prediction confidence is mapped onto the CalpT structure (pLDDT48, predicted local distance difference test). b, A superposition of the predicted CalpT structure (compare Fig. 2b) with one monomer of the MazE/F complex (PDB-IDs: 4ME7)35. The AlphaFold233 prediction confidence is mapped onto the CalpT structure (pLDDT48, predicted local distance difference test). c, Gel filtration chromatography (Superdex 75 16/60) of CalpT The experiment was performed multiple times (n > 3 biological replicates). According to the MALS data in Fig. 3, isolated CalpT behaves as a monomer. Inset: SDS-PAGE analysis of the fractions indicated by the blue bar. d, Peptide fingerprints of cleavage bands. The indicated gel-bands were cut from the gel and submitted for identification at the Mass spectrometry and proteomics facility at the University of St Andrews (Fife, UK, https://mass-spec.wp.st-andrews.ac.uk). Red letters indicate peptides that were identified in the respective sample. The experiment was performed once. e, Mutational analysis of potential CalpL cleavage sites in CalpT. The experiment was performed multiple times (n > 3 technical replicates). For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 5 Characterization of the CalpL/T complex.
a, Single cycle kinetics SPR data of the CalpL/T interaction. The interaction is very strong but cannot be satisfyingly fitted with a 1:1 binding model. The experiment was performed twice (n = 2 technical replicates). b, As a), but an artificial construct of an unspecific VHH fused to CalpT was used as analyte in this experiment. The interaction is very similar to the CalpL/T interaction. The experiment was performed twice (n = 2 technical replicates). c, Schematics of two artificial constructs containing the CalpL cleavage site. d, CalpL cleaves an artificial construct of an unspecific VHH fused to CalpT10 but not a construct of two VHHs fused by the CalpL cleavage site. The experiment was performed once. e, SEC-MALS traces (solid lines: UV280, dashed lines: MWMALS) of proteolysis reactions with different combinations of CalpL S152A, CalpT, and cA4. The schematic indicates the molecular species behind the individual peaks. The experiments were performed twice with slightly different buffer conditions (n = 2 technical replicates). f, Binding of CalpL wt to the indicated CalpT mutants in the absence of cA4. The schematic indicates the position of the mutant in the CalpL/T complex. The experiments were performed once. g, Representative electron density of the CalpL/T10 crystal structure. Selected residues are labeled. The black mesh is a 2mFo-DFc electron density map contoured at 1.0 σ. h, SEC-SAXS experiment of the CalpL/T10 complex. The experiment was performed once. Thirty sample intensity frames and sixty buffer intensity frames were collected and averaged. For each data set and angular point the errors were computed following the Poisson statistics. The data points represent the average intensity difference (sample-buffer) and the error bars represent the standard deviation. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 6 cA4 induced oligomerization of CalpL studied by DLS and SAXS.
a, Dynamic light scattering experiments (six timeseries, each series marked by a dashed circle, single data points are shown) at different protein concentrations and in the absence (t = 0: light grey to t = 60 min: dark grey) and presence (t = 0: cyan to t = 60 min: violet) of cA4 reveal a cA4-dependent oligomerization of CalpL. The experiment was performed twice (n = 2 technical replicates) b, SAXS experiments at different concentrations. The experiments were performed once. For each experiment, thirty sample intensity frames and sixty buffer intensity frames were collected and averaged. For each data set and angular point the errors were computed following the Poisson statistics. The data points represent the average intensity difference (sample-buffer) and the error bars represent the standard deviation. c, Ab initio/rigid-body model of a CalpL dimer created with DAMMIF and SASREFMX by a global fit of a monomer-dimer mixture to the different concentrations (red lines). The crystal structure of the CalpL monomer is shown on the same scale.
Extended Data Fig. 7 Probing the RNase activity of the activated toxin and checking for cA4 induced dimerization of CalpT with pulsed EPR.
a, Fluorescence image of the denaturing PAGE to determine ribonuclease activity of the reactions in b) against six fluorescently labelled RNA substrates (listed in c)). No cleavage was observed after 30 min incubation with RNAs at 60 °C. The experiment was performed three times (n = 3 biological replicates) b, SDS-PAGE analysis of cA4-induced cleavage of CalpT (33 kDa) by CalpL. Cleavage is complete after 60 min at 60 °C. The experiment was performed three times (n = 3 biological replicates) c, Sequences of the RNA substrates d, left: MazF was incubated with a single stranded RNA library containing 10 random bases. Illumina sequencing was used to check for sequences that were cleaved by MazF. Compared to a control reaction without MazF, sequences containing the known MazF target site (ACA) were depleted. right: same experiment but with CalpL/T ± cA4 instead of MazF. No off-diagonal sequences and hence no ssRNase activity were observed. The experiment was performed two times (n = 2 biological replicates). e, Oligonucleotides for the experiments in d) f, AlphaFold2 dimer models of CalpT23. g, Best model (pLDDT48, predicted local distance difference test) including MTSSL spin label80. h, X-band cw-EPR spectrum of of CalpL/T E119R1. The amount of free label (sharp spikes) is ~10%. The labelling efficiency determined as ~100%. i, PELDOR time traces of CalpL/T E119R1 in the presence (red) and absence (black) of cA4. j, Consensus distributions and corresponding uncertainty bands. Colored bars indicate reliability ranges (green: shape reliable; yellow: mean and width reliable; orange: mean reliable; red: no quantification possible). Predicted distance calculated with mtsslWizard80. The EPR experiment was performed twice (n = 2 technical replicates). For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 8 AlphaFold2 predictions of CalpS.
a, Prediction of CalpS alone. The protein is shown as cartoon and colored according to the prediction confidence (pLDDT48, predicted local distance difference test) b, Prediction of the CalpT/S complex. c, Superposition of CalpS with 4LUP81 and 2H2782 identify the DNA binding regions of CalpS. d, Model of CalpS in the context of a RNAP/ECF σ-factor/promotor complex (PDB: 5ZX283, grey, yellow, green) from M. tuberculosis. Note that the linker region between the σ2 and σ4 subunits of CalpS has been cut to allow the superposition of the σ2 and σ4 domains onto those of 5ZX2. The linker is long enough to bind to the RNAP in a similar way as the σ-factor in the 5ZX2 structure (yellow).
Supplementary information
Supplementary Fig. 1
Uncropped and unedited versions of all gels used in this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rouillon, C., Schneberger, N., Chi, H. et al. Antiviral signalling by a cyclic nucleotide activated CRISPR protease. Nature 614, 168–174 (2023). https://doi.org/10.1038/s41586-022-05571-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05571-7
This article is cited by
-
CRISPR technologies for genome, epigenome and transcriptome editing
Nature Reviews Molecular Cell Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.